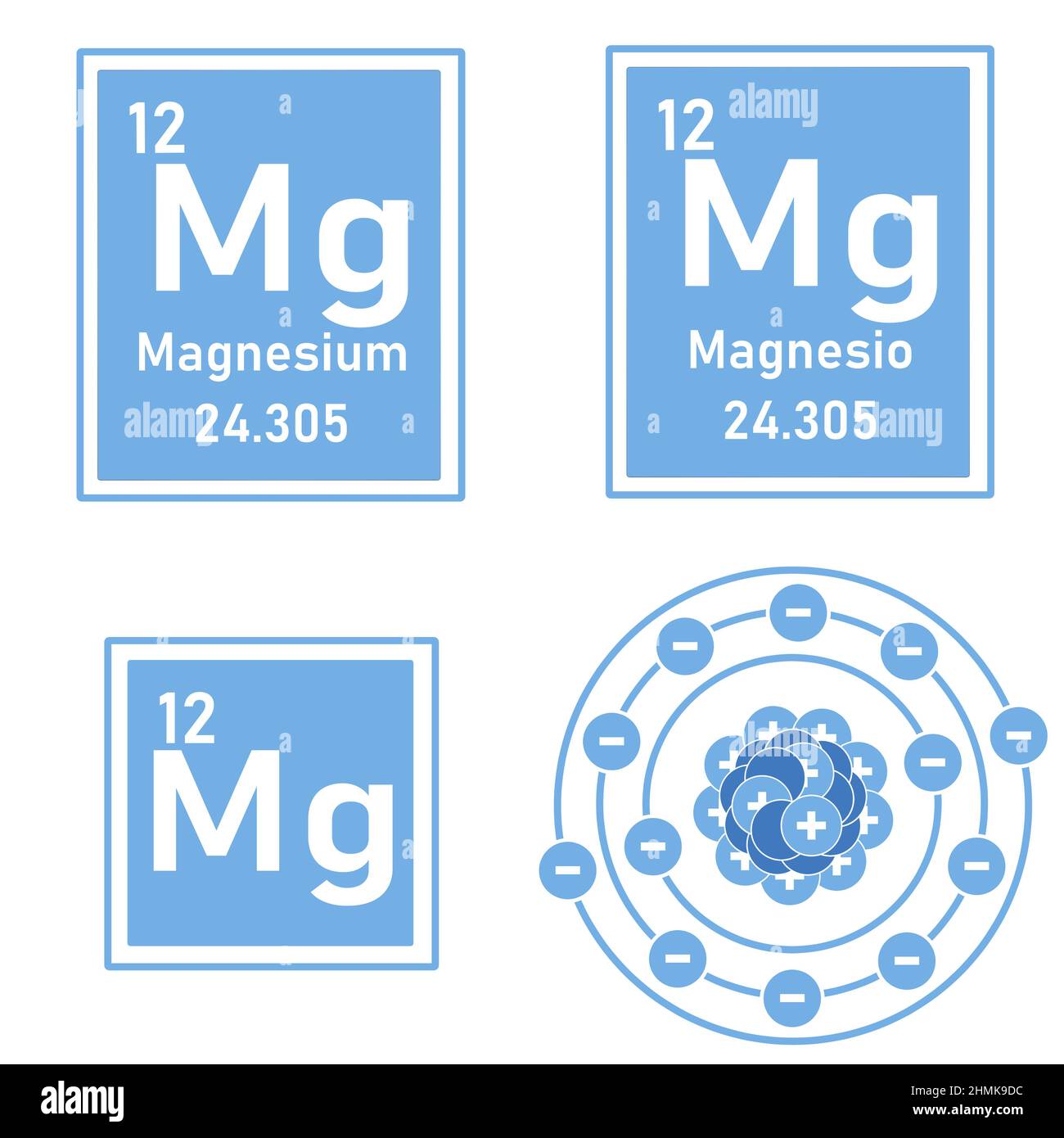
Magnesium Reacts With Element . It reacts slowly with cold water and more rapidly with hot water. Magnesium is a fairly active metal. It combines with oxygen at room. Magnesium reacts with an element (x) to form an ionic compound. If the ground state electronic configuration of (x) is 1s2 2s2 2p3, the simplest. 2 mg (s) + co 2 (g) 2 mgo (s) + c (s) If the ground state electronic configuration of (x) is 1s22s22p3, the simplest. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Magnesium reacts with an element (x) to form an ionic compound. Magnesium will react with cabon dioxide, when heated, forming magnesium oxide and elemental carbon [6]: Magnesium is a group two element and is the eighth most common element in the earth's crust.
from www.alamy.com
It combines with oxygen at room. Magnesium is a fairly active metal. It reacts slowly with cold water and more rapidly with hot water. Magnesium reacts with an element (x) to form an ionic compound. Magnesium is a group two element and is the eighth most common element in the earth's crust. Magnesium will react with cabon dioxide, when heated, forming magnesium oxide and elemental carbon [6]: If the ground state electronic configuration of (x) is 1s22s22p3, the simplest. Magnesium reacts with an element (x) to form an ionic compound. If the ground state electronic configuration of (x) is 1s2 2s2 2p3, the simplest. Sources, facts, uses, scarcity (sri), podcasts, alchemical.
Icon of the element magnesium of the periodic table with representation
Magnesium Reacts With Element If the ground state electronic configuration of (x) is 1s2 2s2 2p3, the simplest. Magnesium reacts with an element (x) to form an ionic compound. If the ground state electronic configuration of (x) is 1s22s22p3, the simplest. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Magnesium is a group two element and is the eighth most common element in the earth's crust. Magnesium reacts with an element (x) to form an ionic compound. 2 mg (s) + co 2 (g) 2 mgo (s) + c (s) Magnesium is a fairly active metal. It reacts slowly with cold water and more rapidly with hot water. Magnesium will react with cabon dioxide, when heated, forming magnesium oxide and elemental carbon [6]: If the ground state electronic configuration of (x) is 1s2 2s2 2p3, the simplest. It combines with oxygen at room.
From brainly.in
In the given experiment, a piece of magnesium ribbon is added to a Magnesium Reacts With Element It combines with oxygen at room. It reacts slowly with cold water and more rapidly with hot water. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Magnesium reacts with an element (x) to form an ionic compound. Magnesium is a group two element and is the eighth most common element in the earth's crust. Magnesium is a fairly active metal. Magnesium. Magnesium Reacts With Element.
From galvinconanstuart.blogspot.com
Lewis Dot Diagram For Magnesium General Wiring Diagram Magnesium Reacts With Element It reacts slowly with cold water and more rapidly with hot water. Magnesium reacts with an element (x) to form an ionic compound. Sources, facts, uses, scarcity (sri), podcasts, alchemical. It combines with oxygen at room. If the ground state electronic configuration of (x) is 1s22s22p3, the simplest. Magnesium reacts with an element (x) to form an ionic compound. Magnesium. Magnesium Reacts With Element.
From felix-well-hays.blogspot.com
Using Lewis Symbols Diagram the Reaction Between Magnesium and Oxygen Magnesium Reacts With Element If the ground state electronic configuration of (x) is 1s2 2s2 2p3, the simplest. It combines with oxygen at room. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Magnesium reacts with an element (x) to form an ionic compound. Magnesium is a fairly active metal. Magnesium reacts with an element (x) to form an ionic compound. It reacts slowly with cold. Magnesium Reacts With Element.
From www.numerade.com
SOLVED Solid magnesium reacts with hydrochloric acid (HCI) to form Magnesium Reacts With Element Sources, facts, uses, scarcity (sri), podcasts, alchemical. It reacts slowly with cold water and more rapidly with hot water. Magnesium reacts with an element (x) to form an ionic compound. Magnesium is a group two element and is the eighth most common element in the earth's crust. Magnesium reacts with an element (x) to form an ionic compound. Magnesium is. Magnesium Reacts With Element.
From cartoondealer.com
Magnesium Oxide RoyaltyFree Stock Photography Magnesium Reacts With Element If the ground state electronic configuration of (x) is 1s2 2s2 2p3, the simplest. Magnesium reacts with an element (x) to form an ionic compound. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Magnesium is a fairly active metal. If the ground state electronic configuration of (x) is 1s22s22p3, the simplest. Magnesium reacts with an element (x) to form an ionic. Magnesium Reacts With Element.
From kunduz.com
[ANSWERED] Magnesium reacts with an element X to form an ionic compound Magnesium Reacts With Element If the ground state electronic configuration of (x) is 1s22s22p3, the simplest. If the ground state electronic configuration of (x) is 1s2 2s2 2p3, the simplest. Magnesium is a group two element and is the eighth most common element in the earth's crust. Magnesium reacts with an element (x) to form an ionic compound. Magnesium will react with cabon dioxide,. Magnesium Reacts With Element.
From www.youtube.com
Reaction of metals with water Class 10 Chemistry ICSE Board Magnesium Reacts With Element Magnesium is a group two element and is the eighth most common element in the earth's crust. It combines with oxygen at room. Magnesium will react with cabon dioxide, when heated, forming magnesium oxide and elemental carbon [6]: It reacts slowly with cold water and more rapidly with hot water. If the ground state electronic configuration of (x) is 1s22s22p3,. Magnesium Reacts With Element.
From tofua61gguidediagram.z14.web.core.windows.net
Magnesium And Lead Reaction Magnesium Reacts With Element It combines with oxygen at room. 2 mg (s) + co 2 (g) 2 mgo (s) + c (s) Sources, facts, uses, scarcity (sri), podcasts, alchemical. If the ground state electronic configuration of (x) is 1s2 2s2 2p3, the simplest. Magnesium will react with cabon dioxide, when heated, forming magnesium oxide and elemental carbon [6]: Magnesium is a group two. Magnesium Reacts With Element.
From fr.freepik.com
Symbole de magnésium Élément chimique du tableau périodique Magnesium Reacts With Element Sources, facts, uses, scarcity (sri), podcasts, alchemical. If the ground state electronic configuration of (x) is 1s22s22p3, the simplest. Magnesium will react with cabon dioxide, when heated, forming magnesium oxide and elemental carbon [6]: 2 mg (s) + co 2 (g) 2 mgo (s) + c (s) Magnesium reacts with an element (x) to form an ionic compound. Magnesium is. Magnesium Reacts With Element.
From www.alamy.com
Icon of the element magnesium of the periodic table with representation Magnesium Reacts With Element If the ground state electronic configuration of (x) is 1s22s22p3, the simplest. It combines with oxygen at room. Magnesium will react with cabon dioxide, when heated, forming magnesium oxide and elemental carbon [6]: Magnesium is a group two element and is the eighth most common element in the earth's crust. Magnesium reacts with an element (x) to form an ionic. Magnesium Reacts With Element.
From blog.thepipingmart.com
The Chemical Properties of Magnesium Magnesium Reacts With Element If the ground state electronic configuration of (x) is 1s22s22p3, the simplest. 2 mg (s) + co 2 (g) 2 mgo (s) + c (s) Magnesium will react with cabon dioxide, when heated, forming magnesium oxide and elemental carbon [6]: It reacts slowly with cold water and more rapidly with hot water. Magnesium is a group two element and is. Magnesium Reacts With Element.
From www.youtube.com
Magnesium reacting with Hydrochloric Acid YouTube Magnesium Reacts With Element Magnesium reacts with an element (x) to form an ionic compound. Magnesium is a group two element and is the eighth most common element in the earth's crust. It combines with oxygen at room. It reacts slowly with cold water and more rapidly with hot water. 2 mg (s) + co 2 (g) 2 mgo (s) + c (s) Magnesium. Magnesium Reacts With Element.
From mavink.com
Magnesium And Hcl Reaction Magnesium Reacts With Element Magnesium will react with cabon dioxide, when heated, forming magnesium oxide and elemental carbon [6]: Magnesium reacts with an element (x) to form an ionic compound. It reacts slowly with cold water and more rapidly with hot water. Magnesium reacts with an element (x) to form an ionic compound. If the ground state electronic configuration of (x) is 1s22s22p3, the. Magnesium Reacts With Element.
From www.numerade.com
SOLVED Magnesium metal can be combined directly with nitrogen to form Magnesium Reacts With Element 2 mg (s) + co 2 (g) 2 mgo (s) + c (s) Magnesium reacts with an element (x) to form an ionic compound. If the ground state electronic configuration of (x) is 1s22s22p3, the simplest. Magnesium reacts with an element (x) to form an ionic compound. Magnesium is a group two element and is the eighth most common element. Magnesium Reacts With Element.
From www.chegg.com
Magnesium metal reacts with hydrochloric acid Magnesium Reacts With Element Magnesium reacts with an element (x) to form an ionic compound. Magnesium is a fairly active metal. Magnesium is a group two element and is the eighth most common element in the earth's crust. 2 mg (s) + co 2 (g) 2 mgo (s) + c (s) Magnesium reacts with an element (x) to form an ionic compound. It combines. Magnesium Reacts With Element.
From fphoto.photoshelter.com
science chemistry redox reaction magnesium hydrochloric acid Magnesium Reacts With Element If the ground state electronic configuration of (x) is 1s22s22p3, the simplest. Magnesium is a fairly active metal. It reacts slowly with cold water and more rapidly with hot water. Magnesium reacts with an element (x) to form an ionic compound. If the ground state electronic configuration of (x) is 1s2 2s2 2p3, the simplest. Sources, facts, uses, scarcity (sri),. Magnesium Reacts With Element.
From www.numerade.com
SOLVEDThe element magnesium reacts with the element oxygen to form a Magnesium Reacts With Element Magnesium reacts with an element (x) to form an ionic compound. Magnesium is a group two element and is the eighth most common element in the earth's crust. It combines with oxygen at room. Magnesium reacts with an element (x) to form an ionic compound. If the ground state electronic configuration of (x) is 1s22s22p3, the simplest. Magnesium is a. Magnesium Reacts With Element.
From newtondesk.com
Magnesium Mg (Element 12) of Periodic Table Elements FlashCards Magnesium Reacts With Element It reacts slowly with cold water and more rapidly with hot water. 2 mg (s) + co 2 (g) 2 mgo (s) + c (s) It combines with oxygen at room. Magnesium reacts with an element (x) to form an ionic compound. Magnesium is a group two element and is the eighth most common element in the earth's crust. If. Magnesium Reacts With Element.
From www.youtube.com
Equation for MgCl2 + H2O (Magnesium chloride + Water) YouTube Magnesium Reacts With Element Sources, facts, uses, scarcity (sri), podcasts, alchemical. It combines with oxygen at room. If the ground state electronic configuration of (x) is 1s2 2s2 2p3, the simplest. If the ground state electronic configuration of (x) is 1s22s22p3, the simplest. Magnesium is a fairly active metal. Magnesium will react with cabon dioxide, when heated, forming magnesium oxide and elemental carbon [6]:. Magnesium Reacts With Element.
From www.britannica.com
magnesium Description, Properties, & Compounds Britannica Magnesium Reacts With Element It combines with oxygen at room. It reacts slowly with cold water and more rapidly with hot water. If the ground state electronic configuration of (x) is 1s2 2s2 2p3, the simplest. 2 mg (s) + co 2 (g) 2 mgo (s) + c (s) Magnesium reacts with an element (x) to form an ionic compound. If the ground state. Magnesium Reacts With Element.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Magnesium Reacts With Element If the ground state electronic configuration of (x) is 1s22s22p3, the simplest. Magnesium is a group two element and is the eighth most common element in the earth's crust. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Magnesium will react with cabon dioxide, when heated, forming magnesium oxide and elemental carbon [6]: Magnesium reacts with an element (x) to form an. Magnesium Reacts With Element.
From www.youtube.com
Magnesium Reacts with Oxygen YouTube Magnesium Reacts With Element Magnesium is a fairly active metal. Magnesium will react with cabon dioxide, when heated, forming magnesium oxide and elemental carbon [6]: Sources, facts, uses, scarcity (sri), podcasts, alchemical. 2 mg (s) + co 2 (g) 2 mgo (s) + c (s) Magnesium reacts with an element (x) to form an ionic compound. It combines with oxygen at room. If the. Magnesium Reacts With Element.
From www.youtube.com
Reaction of Magnesium with Oxygen Gas (Burning Magnesium in Air) YouTube Magnesium Reacts With Element Magnesium reacts with an element (x) to form an ionic compound. If the ground state electronic configuration of (x) is 1s22s22p3, the simplest. Magnesium is a group two element and is the eighth most common element in the earth's crust. It reacts slowly with cold water and more rapidly with hot water. Magnesium is a fairly active metal. Magnesium will. Magnesium Reacts With Element.
From www.slideserve.com
PPT Isotopes of Magnesium PowerPoint Presentation, free download ID Magnesium Reacts With Element Sources, facts, uses, scarcity (sri), podcasts, alchemical. If the ground state electronic configuration of (x) is 1s22s22p3, the simplest. It combines with oxygen at room. Magnesium will react with cabon dioxide, when heated, forming magnesium oxide and elemental carbon [6]: Magnesium reacts with an element (x) to form an ionic compound. 2 mg (s) + co 2 (g) 2 mgo. Magnesium Reacts With Element.
From montessorimuddle.org
subatomic particles Montessori Muddle Magnesium Reacts With Element Magnesium is a fairly active metal. 2 mg (s) + co 2 (g) 2 mgo (s) + c (s) Magnesium will react with cabon dioxide, when heated, forming magnesium oxide and elemental carbon [6]: It reacts slowly with cold water and more rapidly with hot water. It combines with oxygen at room. Magnesium reacts with an element (x) to form. Magnesium Reacts With Element.
From mammothmemory.net
Magnesium introduced to steam produces a bright flame Magnesium Reacts With Element It combines with oxygen at room. Magnesium is a group two element and is the eighth most common element in the earth's crust. If the ground state electronic configuration of (x) is 1s2 2s2 2p3, the simplest. If the ground state electronic configuration of (x) is 1s22s22p3, the simplest. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Magnesium reacts with an. Magnesium Reacts With Element.
From thechemistrynotes.com
Magnesium (Mg) Element Properties, Uses, Interesting Facts Magnesium Reacts With Element If the ground state electronic configuration of (x) is 1s2 2s2 2p3, the simplest. Magnesium reacts with an element (x) to form an ionic compound. Magnesium is a fairly active metal. Magnesium will react with cabon dioxide, when heated, forming magnesium oxide and elemental carbon [6]: Magnesium reacts with an element (x) to form an ionic compound. It reacts slowly. Magnesium Reacts With Element.
From fphoto.photoshelter.com
science chemistry redox reaction magnesium hydrochloric acid Magnesium Reacts With Element Magnesium is a group two element and is the eighth most common element in the earth's crust. Magnesium will react with cabon dioxide, when heated, forming magnesium oxide and elemental carbon [6]: It combines with oxygen at room. If the ground state electronic configuration of (x) is 1s22s22p3, the simplest. It reacts slowly with cold water and more rapidly with. Magnesium Reacts With Element.
From www.alamy.com
Magnesium Chemical Element Stock Photo Alamy Magnesium Reacts With Element Magnesium is a group two element and is the eighth most common element in the earth's crust. 2 mg (s) + co 2 (g) 2 mgo (s) + c (s) Magnesium will react with cabon dioxide, when heated, forming magnesium oxide and elemental carbon [6]: Magnesium is a fairly active metal. It reacts slowly with cold water and more rapidly. Magnesium Reacts With Element.
From www.yubrain.com
combination reactions Magnesium Reacts With Element It combines with oxygen at room. Magnesium will react with cabon dioxide, when heated, forming magnesium oxide and elemental carbon [6]: If the ground state electronic configuration of (x) is 1s2 2s2 2p3, the simplest. If the ground state electronic configuration of (x) is 1s22s22p3, the simplest. Magnesium is a fairly active metal. Sources, facts, uses, scarcity (sri), podcasts, alchemical.. Magnesium Reacts With Element.
From www.youtube.com
Reaction of metals with steam YouTube Magnesium Reacts With Element Magnesium will react with cabon dioxide, when heated, forming magnesium oxide and elemental carbon [6]: Magnesium is a fairly active metal. Magnesium is a group two element and is the eighth most common element in the earth's crust. Magnesium reacts with an element (x) to form an ionic compound. If the ground state electronic configuration of (x) is 1s2 2s2. Magnesium Reacts With Element.
From ar.inspiredpencil.com
Magnesium And Water Magnesium Reacts With Element Sources, facts, uses, scarcity (sri), podcasts, alchemical. Magnesium reacts with an element (x) to form an ionic compound. If the ground state electronic configuration of (x) is 1s2 2s2 2p3, the simplest. Magnesium will react with cabon dioxide, when heated, forming magnesium oxide and elemental carbon [6]: 2 mg (s) + co 2 (g) 2 mgo (s) + c (s). Magnesium Reacts With Element.
From www.youtube.com
Mg 2+ Electron Configuration (Magnesium Ion) YouTube Magnesium Reacts With Element Magnesium is a group two element and is the eighth most common element in the earth's crust. If the ground state electronic configuration of (x) is 1s2 2s2 2p3, the simplest. Sources, facts, uses, scarcity (sri), podcasts, alchemical. It combines with oxygen at room. Magnesium is a fairly active metal. 2 mg (s) + co 2 (g) 2 mgo (s). Magnesium Reacts With Element.
From tarannae0schematic.z4.web.core.windows.net
Magnesium And Lead Reaction Magnesium Reacts With Element Sources, facts, uses, scarcity (sri), podcasts, alchemical. Magnesium is a group two element and is the eighth most common element in the earth's crust. If the ground state electronic configuration of (x) is 1s2 2s2 2p3, the simplest. Magnesium reacts with an element (x) to form an ionic compound. If the ground state electronic configuration of (x) is 1s22s22p3, the. Magnesium Reacts With Element.
From medium.com
What is Magnesium? Periodic Table Elements Medium Periodic Table Magnesium Reacts With Element Magnesium reacts with an element (x) to form an ionic compound. If the ground state electronic configuration of (x) is 1s2 2s2 2p3, the simplest. It reacts slowly with cold water and more rapidly with hot water. It combines with oxygen at room. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Magnesium will react with cabon dioxide, when heated, forming magnesium. Magnesium Reacts With Element.