What Is An Equivalence Point Titration . A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. After hydrolysis is complete, the leftover base is titrated to determine how much was needed to hydrolyze the fat sample. In a titration, if the base. In an ideal world, the colour change would happen when you mix the two solutions together in the correct proportions according to the equation. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions.
from www.chegg.com
In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. In a titration, if the base. In an ideal world, the colour change would happen when you mix the two solutions together in the correct proportions according to the equation. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. After hydrolysis is complete, the leftover base is titrated to determine how much was needed to hydrolyze the fat sample. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte.
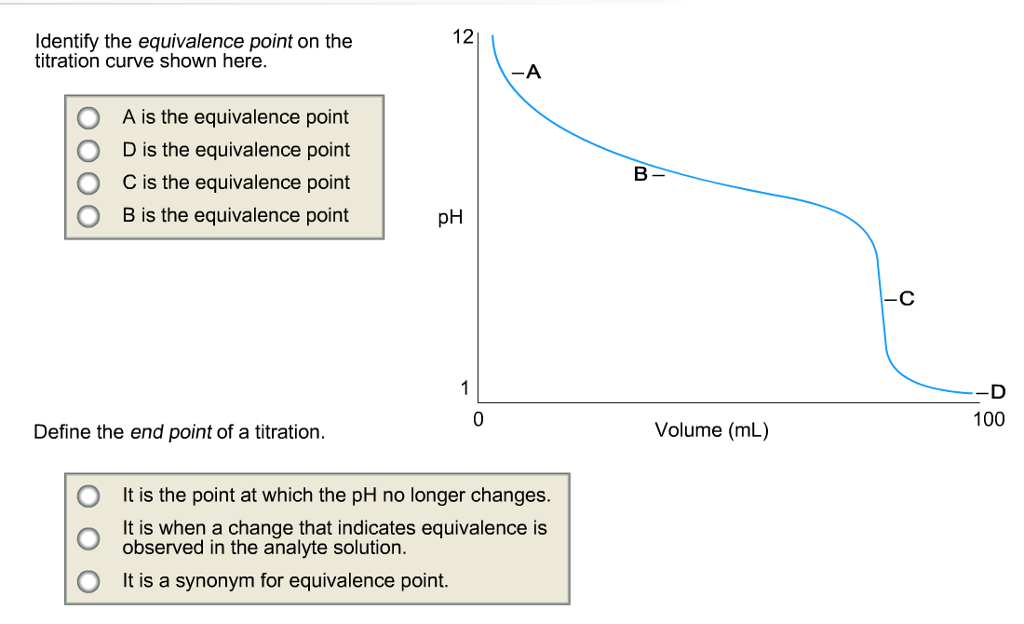
Solved Identify the equivalence point on the titration curve
What Is An Equivalence Point Titration In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. After hydrolysis is complete, the leftover base is titrated to determine how much was needed to hydrolyze the fat sample. In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. In a titration, if the base. In an ideal world, the colour change would happen when you mix the two solutions together in the correct proportions according to the equation. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 What Is An Equivalence Point Titration In a titration, if the base. In an ideal world, the colour change would happen when you mix the two solutions together in the correct proportions according to the equation. After hydrolysis is complete, the leftover base is titrated to determine how much was needed to hydrolyze the fat sample. A point of equivalence in a titration refers to a. What Is An Equivalence Point Titration.
From exoaqbcwf.blob.core.windows.net
Equivalence Point Titration With Base at Alvin Barajas blog What Is An Equivalence Point Titration In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. In a titration, if the base. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In an ideal world, the. What Is An Equivalence Point Titration.
From studydystrophic.z4.web.core.windows.net
How To Find Equivalence Point On Excel Graph What Is An Equivalence Point Titration In an ideal world, the colour change would happen when you mix the two solutions together in the correct proportions according to the equation. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The equivalence point of a chemical reaction is the point at which equal. What Is An Equivalence Point Titration.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts What Is An Equivalence Point Titration After hydrolysis is complete, the leftover base is titrated to determine how much was needed to hydrolyze the fat sample. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In a titration, if the base. In an ideal world, the colour change would happen when you mix the two solutions. What Is An Equivalence Point Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators What Is An Equivalence Point Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. After hydrolysis is complete, the leftover base is titrated to determine how much was needed to hydrolyze the. What Is An Equivalence Point Titration.
From differencesfinder.com
Difference Between Equivalence Point and End Point Differences Finder What Is An Equivalence Point Titration The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. A point of equivalence in a titration refers to a point. What Is An Equivalence Point Titration.
From www.numerade.com
SOLVED The halfequivalence point of a titration occurs half way to What Is An Equivalence Point Titration In an ideal world, the colour change would happen when you mix the two solutions together in the correct proportions according to the equation. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. A point of equivalence in a titration refers to a point at which the added titrant is. What Is An Equivalence Point Titration.
From www.writework.com
Titration of amino acids WriteWork What Is An Equivalence Point Titration In an ideal world, the colour change would happen when you mix the two solutions together in the correct proportions according to the equation. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. After hydrolysis is complete, the leftover base is titrated to determine how much was needed to hydrolyze. What Is An Equivalence Point Titration.
From www.youtube.com
Lecture 7 Titration Half Equivalence Point YouTube What Is An Equivalence Point Titration In a titration, if the base. After hydrolysis is complete, the leftover base is titrated to determine how much was needed to hydrolyze the fat sample. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In an ideal world, the colour change would happen when you mix the two solutions. What Is An Equivalence Point Titration.
From www.youtube.com
Titration curves in details. Equivalence point. Half equivalence point What Is An Equivalence Point Titration In an ideal world, the colour change would happen when you mix the two solutions together in the correct proportions according to the equation. In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. After hydrolysis is complete, the leftover. What Is An Equivalence Point Titration.
From www.youtube.com
Titration Curves, Equivalence Point YouTube What Is An Equivalence Point Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. In a titration, if the base. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In an ideal world, the colour change would happen when you mix. What Is An Equivalence Point Titration.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent What Is An Equivalence Point Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. After hydrolysis is complete, the leftover base is titrated to determine how much was needed to hydrolyze the fat sample. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are. What Is An Equivalence Point Titration.
From mavink.com
What Is The Equivalence Point Of A Titration What Is An Equivalence Point Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. In a titration, if the base. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In an ideal world, the colour change would happen when you mix. What Is An Equivalence Point Titration.
From www.echemi.com
Titration of CH3COONa with HCl and pKa determination from half What Is An Equivalence Point Titration In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. After hydrolysis is complete, the leftover base is titrated to determine how much was needed to hydrolyze the fat sample. A point of equivalence in a titration refers to a. What Is An Equivalence Point Titration.
From www.coursehero.com
[Solved] Calculate the pH at the equivalence point for the titration What Is An Equivalence Point Titration The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. After hydrolysis is complete, the leftover base is titrated to determine how much was needed to hydrolyze the fat sample. In a titration, if the base. In an ideal world, the colour change would happen when you mix the two solutions. What Is An Equivalence Point Titration.
From www.myxxgirl.com
Where Does The Equivalence Point Occur On A Titration My XXX Hot Girl What Is An Equivalence Point Titration After hydrolysis is complete, the leftover base is titrated to determine how much was needed to hydrolyze the fat sample. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have. What Is An Equivalence Point Titration.
From hxeptiewa.blob.core.windows.net
Find Equivalence Point From Titration at Roger Cornelius blog What Is An Equivalence Point Titration In an ideal world, the colour change would happen when you mix the two solutions together in the correct proportions according to the equation. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. After hydrolysis is complete, the leftover base is titrated to determine how much. What Is An Equivalence Point Titration.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki What Is An Equivalence Point Titration In a titration, if the base. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. A point of equivalence in. What Is An Equivalence Point Titration.
From www.chegg.com
Solved Identify the equivalence point on the titration curve What Is An Equivalence Point Titration In a titration, if the base. After hydrolysis is complete, the leftover base is titrated to determine how much was needed to hydrolyze the fat sample. In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. In an ideal world,. What Is An Equivalence Point Titration.
From mungfali.com
Half Equivalence Point On Titration Curve What Is An Equivalence Point Titration In a titration, if the base. After hydrolysis is complete, the leftover base is titrated to determine how much was needed to hydrolyze the fat sample. In an ideal world, the colour change would happen when you mix the two solutions together in the correct proportions according to the equation. The equivalence point of a chemical reaction is the point. What Is An Equivalence Point Titration.
From solvedlib.com
What is a titration? What is the equivalence point o… SolvedLib What Is An Equivalence Point Titration In an ideal world, the colour change would happen when you mix the two solutions together in the correct proportions according to the equation. In a titration, if the base. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. A point of equivalence in a titration refers to a point. What Is An Equivalence Point Titration.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co What Is An Equivalence Point Titration In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. After hydrolysis is complete, the leftover base is titrated to determine how much was needed to hydrolyze the fat sample. In an ideal world, the colour change would happen when. What Is An Equivalence Point Titration.
From mungfali.com
Equivalence Points On Titration Graph What Is An Equivalence Point Titration In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. In a titration, if the base. After hydrolysis is complete, the leftover base is titrated to determine how much was needed to hydrolyze the fat sample. A point of equivalence. What Is An Equivalence Point Titration.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid What Is An Equivalence Point Titration After hydrolysis is complete, the leftover base is titrated to determine how much was needed to hydrolyze the fat sample. In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. A point of equivalence in a titration refers to a. What Is An Equivalence Point Titration.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent What Is An Equivalence Point Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In an ideal world, the colour change would happen when you mix the two solutions together in the. What Is An Equivalence Point Titration.
From socratic.org
The "pH" at onehalf the equivalence point in an acidbase titration What Is An Equivalence Point Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. In an ideal world, the colour change would happen when you mix the two solutions together in the correct proportions according to the equation. After hydrolysis is complete, the leftover base is titrated to determine how much. What Is An Equivalence Point Titration.
From vasasimonmackenzie.blogspot.com
Equivalence Point Titration Simon Mackenzie What Is An Equivalence Point Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In an ideal world, the colour change would happen when you mix the two solutions together in the. What Is An Equivalence Point Titration.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax What Is An Equivalence Point Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. In a titration, if the base. After hydrolysis is complete, the leftover base is titrated to determine how much was needed to hydrolyze the fat sample. The equivalence point of a chemical reaction is the point at. What Is An Equivalence Point Titration.
From www.vrogue.co
Acid Base Titration Titration Curves Equivalence Poin vrogue.co What Is An Equivalence Point Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In a titration, if the base. After hydrolysis is complete, the leftover base is titrated to determine how. What Is An Equivalence Point Titration.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept What Is An Equivalence Point Titration The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. After hydrolysis is complete, the leftover base is titrated to determine. What Is An Equivalence Point Titration.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax What Is An Equivalence Point Titration In an ideal world, the colour change would happen when you mix the two solutions together in the correct proportions according to the equation. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. A point of equivalence in a titration refers to a point at which the added titrant is. What Is An Equivalence Point Titration.
From narodnatribuna.info
Solved The Titration Curve Shown Below Represents A 25 Ml What Is An Equivalence Point Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. In an ideal world, the colour change. What Is An Equivalence Point Titration.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts What Is An Equivalence Point Titration After hydrolysis is complete, the leftover base is titrated to determine how much was needed to hydrolyze the fat sample. In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. In a titration, if the base. In an ideal world,. What Is An Equivalence Point Titration.
From mavink.com
Acid Base Titration Calculation What Is An Equivalence Point Titration In an ideal world, the colour change would happen when you mix the two solutions together in the correct proportions according to the equation. After hydrolysis is complete, the leftover base is titrated to determine how much was needed to hydrolyze the fat sample. A point of equivalence in a titration refers to a point at which the added titrant. What Is An Equivalence Point Titration.
From www.aiophotoz.com
How To Find The Half Equivalence Point In A Titration Graph Sciencing What Is An Equivalence Point Titration In a titration, if the base. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. A point of equivalence in. What Is An Equivalence Point Titration.