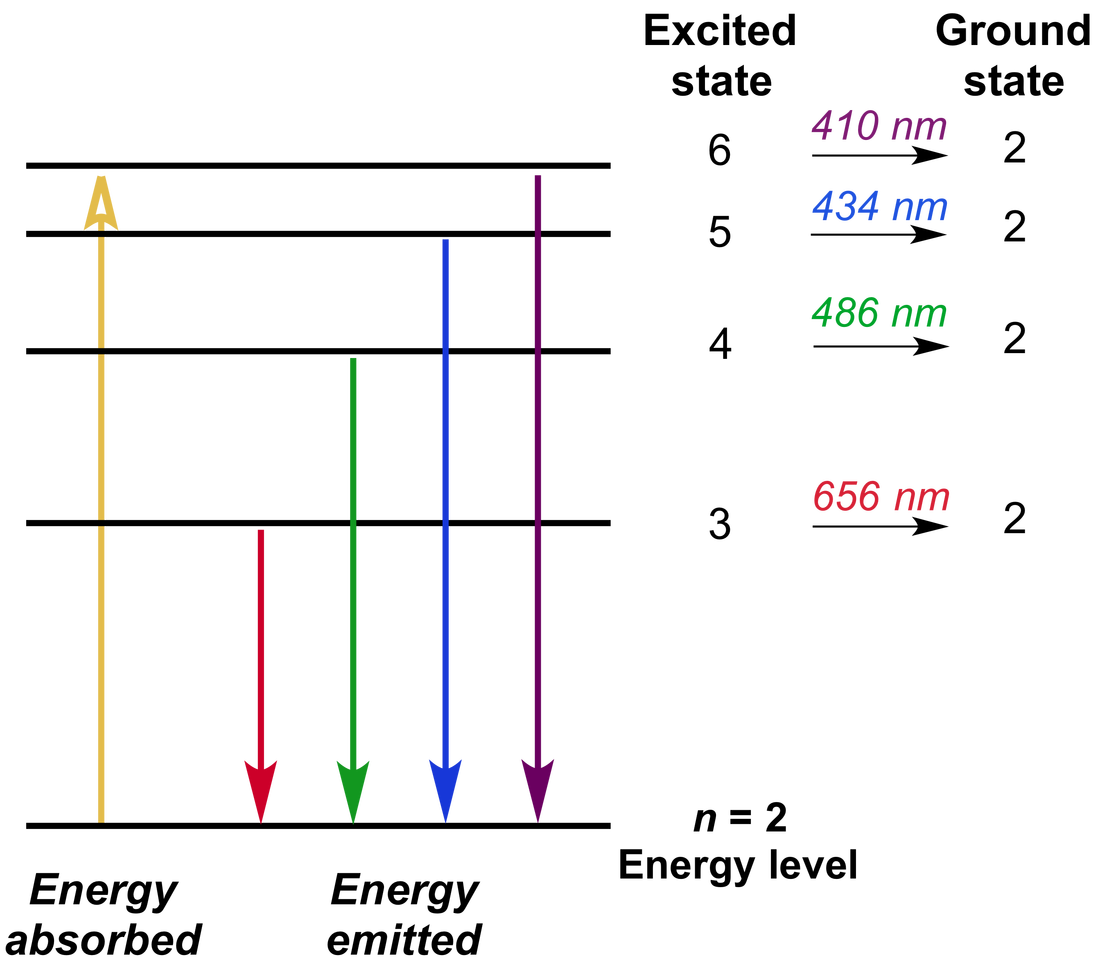
Emission Spectrum Spectroscopy . atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. 10.7.1 atomic emission spectra.
from ceknofbz.blob.core.windows.net
Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. 10.7.1 atomic emission spectra.
Emission Spectra Formula at John Wagner blog
Emission Spectrum Spectroscopy atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. 10.7.1 atomic emission spectra. atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity.
From chem.libretexts.org
5.5 Atomic Emission Spectra Chemistry LibreTexts Emission Spectrum Spectroscopy the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states. Emission Spectrum Spectroscopy.
From fineartamerica.com
Hydrogen Emission And Absorption Spectra Photograph by Carlos Clarivan Emission Spectrum Spectroscopy atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected. Emission Spectrum Spectroscopy.
From cemexerf.blob.core.windows.net
Light Emission Spectroscopy at Claudia Kidd blog Emission Spectrum Spectroscopy Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. 10.7.1 atomic emission spectra. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. atomic emission spectrum results. Emission Spectrum Spectroscopy.
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction Emission Spectrum Spectroscopy 10.7.1 atomic emission spectra. atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. the. Emission Spectrum Spectroscopy.
From www.slideserve.com
PPT Lecture 12 Introduction to Atomic Spectroscopy PowerPoint Emission Spectrum Spectroscopy Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. 10.7.1 atomic emission spectra. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element. Emission Spectrum Spectroscopy.
From sciencephotogallery.com
Helium Emission And Absorption Spectra by Carlos Clarivan Emission Spectrum Spectroscopy atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. Classical emission spectroscopy is based on excitation. Emission Spectrum Spectroscopy.
From courses.lumenlearning.com
Spectroscopy in Astronomy Astronomy Emission Spectrum Spectroscopy Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. 10.7.1 atomic emission spectra. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. atomic emission spectrum results. Emission Spectrum Spectroscopy.
From chem.libretexts.org
10.6 Photoluminescence Spectroscopy Chemistry LibreTexts Emission Spectrum Spectroscopy Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. 10.7.1 atomic emission spectra. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. the emission spectrum (or. Emission Spectrum Spectroscopy.
From spiff.rit.edu
Spectrographs and Spectra Emission Spectrum Spectroscopy 10.7.1 atomic emission spectra. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. atomic emission spectrum results. Emission Spectrum Spectroscopy.
From studylib.net
XRAY EMISSION SPECTRUM Emission Spectrum Spectroscopy atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. Atomic emission occurs when a valence electron. Emission Spectrum Spectroscopy.
From www.scribd.com
Aas Emission Spectrum Atomic Absorption Spectroscopy Emission Spectrum Spectroscopy 10.7.1 atomic emission spectra. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. Classical emission. Emission Spectrum Spectroscopy.
From animalia-life.club
Bohr Model Of Hydrogen Emission Spectrum Emission Spectrum Spectroscopy atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. 10.7.1 atomic emission spectra. the. Emission Spectrum Spectroscopy.
From ceknofbz.blob.core.windows.net
Emission Spectra Formula at John Wagner blog Emission Spectrum Spectroscopy atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. the emission spectrum (or line spectrum). Emission Spectrum Spectroscopy.
From studyposter.blogspot.com
How Is A Stars Emission Spectrum Used To Study Stars Study Poster Emission Spectrum Spectroscopy Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. the emission spectrum (or line spectrum). Emission Spectrum Spectroscopy.
From montessorimuddle.org
Emission Spectra How Atoms Emit and Absorb Light Montessori Muddle Emission Spectrum Spectroscopy atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. atomic emission spectroscopy (aes) deals with the excitation. Emission Spectrum Spectroscopy.
From www.dreamstime.com
Elements Emission Spectrum List Lines Visible Light Spectra Stock Emission Spectrum Spectroscopy 10.7.1 atomic emission spectra. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. Classical emission spectroscopy is based on excitation of atoms. Emission Spectrum Spectroscopy.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Emission Spectrum Spectroscopy the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states. Emission Spectrum Spectroscopy.
From www.researchgate.net
Emission spectra of different light sources (a) incandescent tungsten Emission Spectrum Spectroscopy the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states. Emission Spectrum Spectroscopy.
From es.slideshare.net
Emission spectroscopy Emission Spectrum Spectroscopy the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. 10.7.1 atomic emission spectra. atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. Classical emission spectroscopy is based on excitation of atoms. Emission Spectrum Spectroscopy.
From penrodkur.blogspot.com
Line Spectrum Of Hydrogen Modelo Atómico de Bohr Características y Emission Spectrum Spectroscopy atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. 10.7.1 atomic emission spectra. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. the. Emission Spectrum Spectroscopy.
From byjus.com
Emission spectrum Atomic spectra Chemistry Emission Spectrum Spectroscopy Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. 10.7.1 atomic emission spectra. the. Emission Spectrum Spectroscopy.
From studymind.co.uk
Flame Emission Spectroscopy (GCSE Chemistry) Study Mind Emission Spectrum Spectroscopy Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. the emission spectrum (or line spectrum) of a chemical. Emission Spectrum Spectroscopy.
From www.comsol.com
Calculating the Emission Spectra from Common Light Sources COMSOL Blog Emission Spectrum Spectroscopy Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. Emission Spectrum Spectroscopy.
From www.astronoo.com
Spectroscopy — Astronoo Emission Spectrum Spectroscopy atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. atomic emission spectrum results in atomic transition from quantum. Emission Spectrum Spectroscopy.
From www.researchgate.net
Emission spectra of LED light sources. Download Scientific Diagram Emission Spectrum Spectroscopy atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. 10.7.1 atomic emission spectra. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. atomic emission spectrum results in atomic transition from quantum. Emission Spectrum Spectroscopy.
From vqg1811-700.com
What are Absorption, Excitation and Emission Spectra? Tech Blog Emission Spectrum Spectroscopy Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. 10.7.1 atomic emission spectra. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. the emission spectrum (or. Emission Spectrum Spectroscopy.
From www.visionlearning.com
Atomic Theory II Chemistry Visionlearning Emission Spectrum Spectroscopy Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. 10.7.1 atomic emission spectra. Classical emission. Emission Spectrum Spectroscopy.
From webbtelescope.org
Absorption and Emission Spectra of Various Elements b Emission Spectrum Spectroscopy Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. the emission spectrum (or line spectrum) of a chemical. Emission Spectrum Spectroscopy.
From kuropataq5ystudyquizz.z13.web.core.windows.net
Atomic Spectra Is An Example Of Emission Spectrum Spectroscopy atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns. Emission Spectrum Spectroscopy.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Emission Spectrum Spectroscopy 10.7.1 atomic emission spectra. atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. the. Emission Spectrum Spectroscopy.
From chemwiki.ucdavis.edu
Chapter 2.3 Atomic Spectra and Models of the Atom Chemwiki Emission Spectrum Spectroscopy atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. 10.7.1 atomic emission spectra. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. the emission spectrum (or. Emission Spectrum Spectroscopy.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Emission Spectrum Spectroscopy the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. 10.7.1 atomic emission spectra. atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. Classical emission spectroscopy is based on excitation of atoms. Emission Spectrum Spectroscopy.
From www.resonancescience.org
What is Resonance and Why is it so Important? Emission Spectrum Spectroscopy atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected. Emission Spectrum Spectroscopy.
From www.researchgate.net
Typical optical emission spectra from a CH 4 , b N 2 , and c mixed CH 4 Emission Spectrum Spectroscopy Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. 10.7.1 atomic emission spectra. atomic emission spectroscopy. Emission Spectrum Spectroscopy.
From laderarctic.weebly.com
Emission spectra laderarctic Emission Spectrum Spectroscopy Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. 10.7.1 atomic emission spectra. atomic emission spectrum results in atomic transition from quantum states of higher energy to those of lower energy. atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation. Atomic emission. Emission Spectrum Spectroscopy.