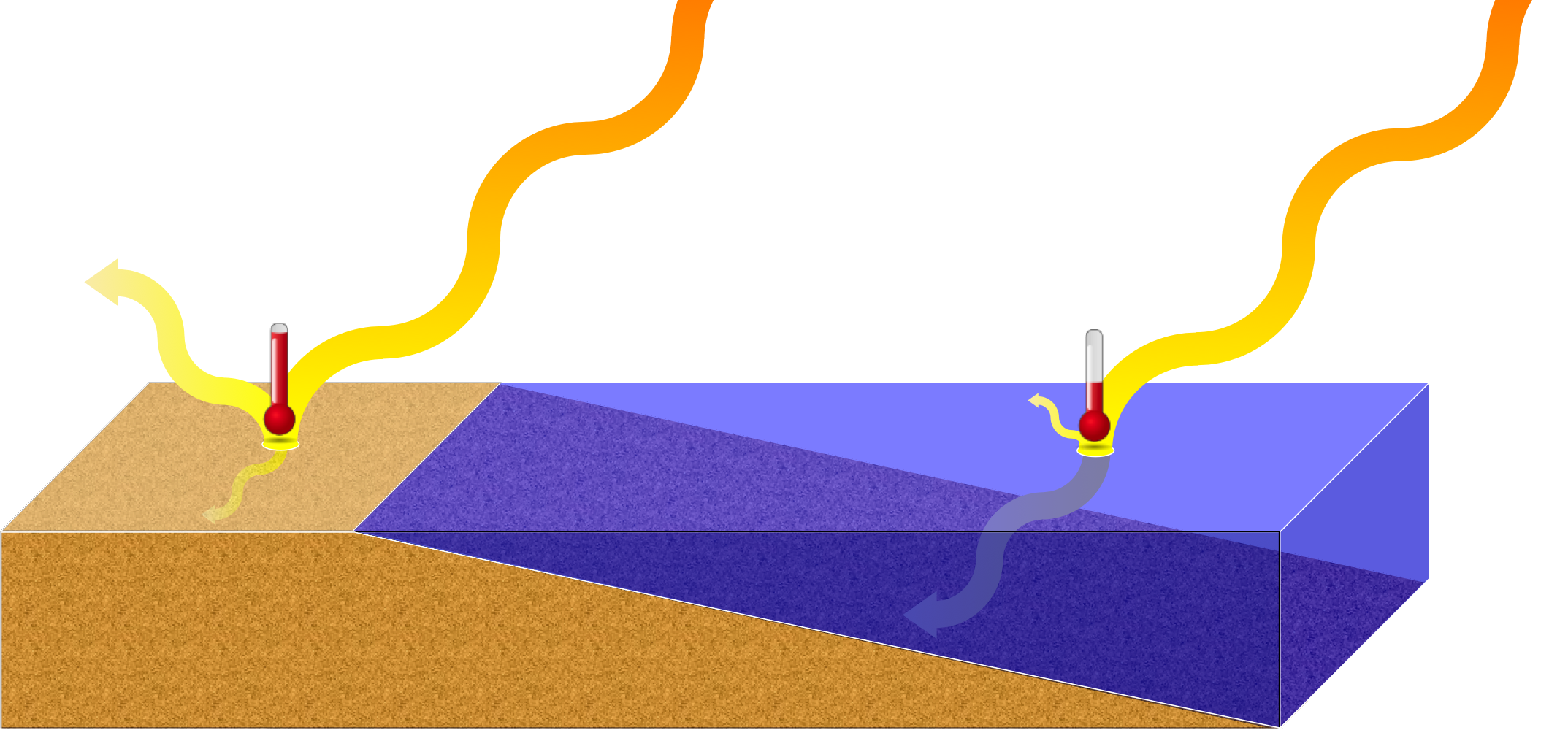
What Is High Heat Capacity In Water . Because water is such an important and common substance, we even have a special way to identify the. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. You may not know how that affects. Specific heat is defined as the amount of heat one gram of a substance must absorb or. Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. The specific heat capacity of a substance is the amount of thermal energy required to raise the temperature of 1kg of that substance by 1°c. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g°c. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Water has a specific heat capacity of 4182 j/kg°c. Water has a particularly high specific heat compared to many other substances. When calculating mass and volume flow in a water heating. Water has the highest specific heat capacity of any liquid.
from thebiologyprimer.com
Water has a specific heat capacity of 4182 j/kg°c. Water has a particularly high specific heat compared to many other substances. You may not know how that affects. Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. The specific heat capacity of a substance is the amount of thermal energy required to raise the temperature of 1kg of that substance by 1°c. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g°c. Water has the highest specific heat capacity of any liquid. Specific heat is defined as the amount of heat one gram of a substance must absorb or. Because water is such an important and common substance, we even have a special way to identify the.
Atoms & Molecules echapter — The Biology Primer
What Is High Heat Capacity In Water For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g°c. Water has a specific heat capacity of 4182 j/kg°c. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Water has a particularly high specific heat compared to many other substances. Because water is such an important and common substance, we even have a special way to identify the. You may not know how that affects. Specific heat is defined as the amount of heat one gram of a substance must absorb or. When calculating mass and volume flow in a water heating. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g°c. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. The specific heat capacity of a substance is the amount of thermal energy required to raise the temperature of 1kg of that substance by 1°c. Water has the highest specific heat capacity of any liquid.
From www.expii.com
High Specific Heat (Water) — Properties & Examples Expii What Is High Heat Capacity In Water The specific heat capacity of a substance is the amount of thermal energy required to raise the temperature of 1kg of that substance by 1°c. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. Water has a specific heat capacity of 4182 j/kg°c. When calculating mass and volume flow in a. What Is High Heat Capacity In Water.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types What Is High Heat Capacity In Water Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. Because water is such an important and common substance, we even have a special way to identify the. Water. What Is High Heat Capacity In Water.
From www.slideserve.com
PPT High Specific Heat of Water PowerPoint Presentation, free What Is High Heat Capacity In Water For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g°c. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. You may not know how that affects. Water has a particularly high specific heat compared to many other substances. When calculating mass and volume. What Is High Heat Capacity In Water.
From www.slideserve.com
PPT Physical Oceanography PowerPoint Presentation, free download ID What Is High Heat Capacity In Water Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. You may not know how that affects. Water has the highest specific heat capacity of any liquid. Because water. What Is High Heat Capacity In Water.
From www.slideserve.com
PPT AP Biology Ch. 3 PowerPoint Presentation, free download ID1989378 What Is High Heat Capacity In Water Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Because water is such an important and common substance, we even have a special way to identify the. When calculating mass and volume flow in a water heating. The specific heat capacity of a substance is the. What Is High Heat Capacity In Water.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID6095414 What Is High Heat Capacity In Water For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g°c. Water has a particularly high specific heat compared to many other substances. Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Water has a specific heat capacity of. What Is High Heat Capacity In Water.
From www.showme.com
Properties of water high heat capacity Science ShowMe What Is High Heat Capacity In Water When calculating mass and volume flow in a water heating. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g°c. The specific heat capacity of a substance is the amount of thermal energy required to raise the temperature of 1kg of that substance by 1°c. Water has the highest specific heat capacity. What Is High Heat Capacity In Water.
From www.slideserve.com
PPT SPECIFIC HEAT CAPACITY PowerPoint Presentation, free download What Is High Heat Capacity In Water When calculating mass and volume flow in a water heating. Water has a specific heat capacity of 4182 j/kg°c. Because water is such an important and common substance, we even have a special way to identify the. Water has a particularly high specific heat compared to many other substances. For liquid at room temperature and pressure, the value of specific. What Is High Heat Capacity In Water.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID6811859 What Is High Heat Capacity In Water For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g°c. Water has the highest specific heat capacity of any liquid. Because water is such an important and common substance, we even have a special way to identify the. Specific heat (c) is the amount of heat required to change the temperature of. What Is High Heat Capacity In Water.
From www.slideserve.com
PPT Define Celsius Scale PowerPoint Presentation ID5751509 What Is High Heat Capacity In Water Water has the highest specific heat capacity of any liquid. Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. You may not know how that affects. Water has a particularly high specific heat compared to many other substances. Water has a high specific heat capacity—it absorbs. What Is High Heat Capacity In Water.
From studylib.net
1.3 Specific Heat Capacity What Is High Heat Capacity In Water You may not know how that affects. The specific heat capacity of a substance is the amount of thermal energy required to raise the temperature of 1kg of that substance by 1°c. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. Water has the highest specific heat capacity of any liquid.. What Is High Heat Capacity In Water.
From www.showme.com
Properties of water high heat capacity Science ShowMe What Is High Heat Capacity In Water For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g°c. Water has the highest specific heat capacity of any liquid. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. The specific heat capacity of a substance is the amount of thermal energy required to raise the temperature. What Is High Heat Capacity In Water.
From www.thoughtco.com
Heat Capacity Definition in Chemistry What Is High Heat Capacity In Water Water has a specific heat capacity of 4182 j/kg°c. Specific heat is defined as the amount of heat one gram of a substance must absorb or. Water has a particularly high specific heat compared to many other substances. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. The specific heat capacity. What Is High Heat Capacity In Water.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer What Is High Heat Capacity In Water Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. Because water is such an important and common substance, we even have a special way to identify the. Water has a particularly high specific heat compared to many other substances. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184. What Is High Heat Capacity In Water.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is High Heat Capacity In Water Water has the highest specific heat capacity of any liquid. The specific heat capacity of a substance is the amount of thermal energy required to raise the temperature of 1kg of that substance by 1°c. Water has a particularly high specific heat compared to many other substances. Specific heat (c) is the amount of heat required to change the temperature. What Is High Heat Capacity In Water.
From www.slideserve.com
PPT LESSON TWO PowerPoint Presentation, free download ID1170548 What Is High Heat Capacity In Water For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g°c. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. The specific heat capacity of a substance is the amount of thermal energy required to raise the temperature of 1kg of that substance by 1°c. Because water is. What Is High Heat Capacity In Water.
From www.geeksforgeeks.org
Heat Capacity Definition, Formula, Unit, Examples, FAQs What Is High Heat Capacity In Water Specific heat is defined as the amount of heat one gram of a substance must absorb or. You may not know how that affects. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. When calculating mass and volume flow in a water heating. Specific heat (c) is the amount of heat. What Is High Heat Capacity In Water.
From studymind.co.uk
Heat Capacity Study Mind What Is High Heat Capacity In Water For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g°c. Because water is such an important and common substance, we even have a special way to identify the. The specific heat capacity of a substance is the amount of thermal energy required to raise the temperature of 1kg of that substance by. What Is High Heat Capacity In Water.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID6095414 What Is High Heat Capacity In Water For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g°c. Specific heat is defined as the amount of heat one gram of a substance must absorb or. The specific heat capacity of a substance is the amount of thermal energy required to raise the temperature of 1kg of that substance by 1°c.. What Is High Heat Capacity In Water.
From slideplayer.com
Water. ppt download What Is High Heat Capacity In Water Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Water has a particularly high specific heat compared to many other substances. For liquid at room temperature and pressure,. What Is High Heat Capacity In Water.
From www.slideserve.com
PPT Notes Water and its Special Properties PowerPoint Presentation What Is High Heat Capacity In Water Water has the highest specific heat capacity of any liquid. Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g°c. Water has a particularly high specific heat compared to. What Is High Heat Capacity In Water.
From www.slideserve.com
PPT PROPERTIES OF WATER PowerPoint Presentation, free download ID What Is High Heat Capacity In Water Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. Specific heat is defined as the amount of heat one gram of a substance must absorb or. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. For liquid at room temperature and pressure, the value of specific. What Is High Heat Capacity In Water.
From www.slideserve.com
PPT High Specific Heat of Water PowerPoint Presentation, free What Is High Heat Capacity In Water You may not know how that affects. Because water is such an important and common substance, we even have a special way to identify the. When calculating mass and volume flow in a water heating. Water has a specific heat capacity of 4182 j/kg°c. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to. What Is High Heat Capacity In Water.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii What Is High Heat Capacity In Water Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Water has a particularly high specific heat compared to many other substances. Specific heat is defined as the amount of heat one gram of a substance must absorb or. When calculating mass and volume flow in a. What Is High Heat Capacity In Water.
From ch301.cm.utexas.edu
heating curve What Is High Heat Capacity In Water Water has the highest specific heat capacity of any liquid. When calculating mass and volume flow in a water heating. Specific heat is defined as the amount of heat one gram of a substance must absorb or. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g°c. The specific heat capacity of. What Is High Heat Capacity In Water.
From classschoolwithstands.z21.web.core.windows.net
High Specific Heat In Water What Is High Heat Capacity In Water Water has a particularly high specific heat compared to many other substances. Specific heat is defined as the amount of heat one gram of a substance must absorb or. Water has a specific heat capacity of 4182 j/kg°c. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. Water has the highest. What Is High Heat Capacity In Water.
From www.slideserve.com
PPT Unit 6 Chemical Properties of Water PowerPoint Presentation What Is High Heat Capacity In Water You may not know how that affects. Specific heat is defined as the amount of heat one gram of a substance must absorb or. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g°c.. What Is High Heat Capacity In Water.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii What Is High Heat Capacity In Water Water has the highest specific heat capacity of any liquid. Because water is such an important and common substance, we even have a special way to identify the. Specific heat is defined as the amount of heat one gram of a substance must absorb or. The specific heat capacity of a substance is the amount of thermal energy required to. What Is High Heat Capacity In Water.
From www.animalia-life.club
Heat Capacity Of Water What Is High Heat Capacity In Water Water has the highest specific heat capacity of any liquid. Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Water has a specific heat capacity of 4182 j/kg°c. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. For liquid at room. What Is High Heat Capacity In Water.
From www.slideserve.com
PPT Atmospheric Moisture PowerPoint Presentation, free download ID What Is High Heat Capacity In Water For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g°c. Because water is such an important and common substance, we even have a special way to identify the. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Water has a high specific heat capacity—it absorbs a lot. What Is High Heat Capacity In Water.
From www.geeksforgeeks.org
Specific Heat Capacity Definition, Formula, Water Heat Capacity What Is High Heat Capacity In Water For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g°c. You may not know how that affects. The specific heat capacity of a substance is the amount of thermal energy required to raise the temperature of 1kg of that substance by 1°c. Its specific heat capacity is 4.184 j/g°c, which means it. What Is High Heat Capacity In Water.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID6095414 What Is High Heat Capacity In Water Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Water has a specific heat. What Is High Heat Capacity In Water.
From pressbooks.bccampus.ca
Water has a high heat capacity Chemistry for Biology 1190 Students What Is High Heat Capacity In Water The specific heat capacity of a substance is the amount of thermal energy required to raise the temperature of 1kg of that substance by 1°c. Water has the highest specific heat capacity of any liquid. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g°c. Water has a particularly high specific heat. What Is High Heat Capacity In Water.
From www.slideserve.com
PPT Explain why water has a high heat capacity. Why is this important What Is High Heat Capacity In Water When calculating mass and volume flow in a water heating. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is approximately 4.2 j/g°c. Water has the highest specific heat capacity of any liquid. Specific heat is defined as the amount of heat one gram of a substance must absorb or. The specific heat capacity of. What Is High Heat Capacity In Water.
From en.ppt-online.org
Class water online presentation What Is High Heat Capacity In Water Water has a particularly high specific heat compared to many other substances. Water has a specific heat capacity of 4182 j/kg°c. Because water is such an important and common substance, we even have a special way to identify the. Specific heat is defined as the amount of heat one gram of a substance must absorb or. You may not know. What Is High Heat Capacity In Water.