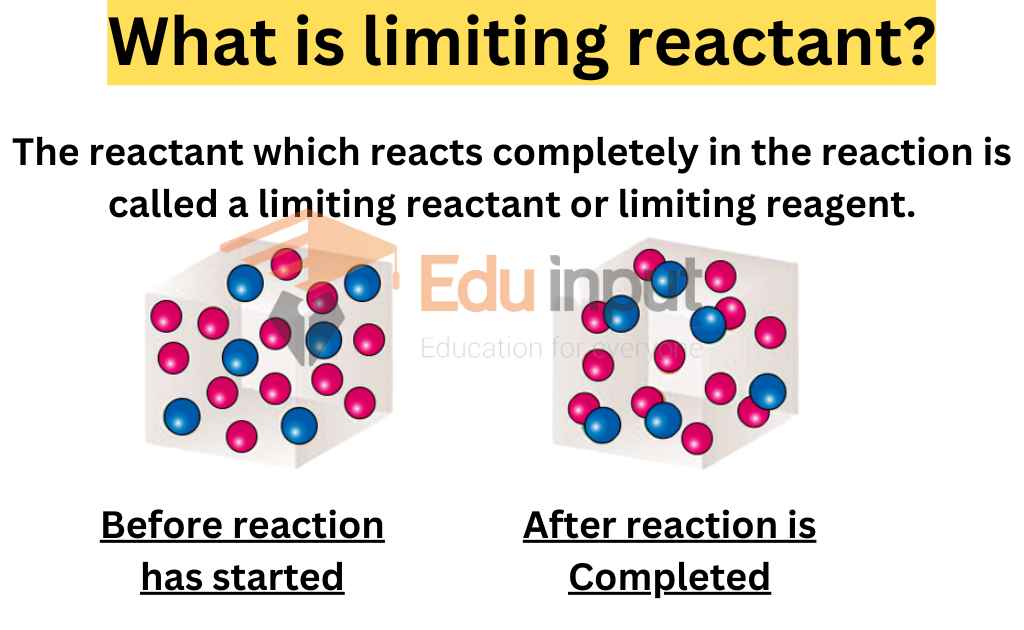
Limiting Reactant Hol Lab . To identify the limiting reactant, calculate the number of moles. The chemical that is used up is called the limiting reactant while the other reactant is present in excess. If both reactants are present in exactly. Determine the limiting reactant and calculate the theoretical yield of pbi 2. The procedure of this experiment requires us to use the hol required items and use the. Determine the limiting reactant in the chemical reaction and use measured data to determine how it affects the amount of product. The reactant you run out of is called the limiting reagent; In the following experiment trials are done with two different constants to determine the limiting reagents for each reaction are both poles of the. Therefore lead (ii) nitrate is the limiting reactant and potassium iodide is in excess. The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. The other reactant or reactants are considered to be in excess.
from eduinput.com
Determine the limiting reactant and calculate the theoretical yield of pbi 2. The chemical that is used up is called the limiting reactant while the other reactant is present in excess. In the following experiment trials are done with two different constants to determine the limiting reagents for each reaction are both poles of the. The procedure of this experiment requires us to use the hol required items and use the. The other reactant or reactants are considered to be in excess. If both reactants are present in exactly. The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. Therefore lead (ii) nitrate is the limiting reactant and potassium iodide is in excess. Determine the limiting reactant in the chemical reaction and use measured data to determine how it affects the amount of product. To identify the limiting reactant, calculate the number of moles.
What is the limiting reactant? Explained with Examples
Limiting Reactant Hol Lab In the following experiment trials are done with two different constants to determine the limiting reagents for each reaction are both poles of the. In the following experiment trials are done with two different constants to determine the limiting reagents for each reaction are both poles of the. To identify the limiting reactant, calculate the number of moles. Determine the limiting reactant in the chemical reaction and use measured data to determine how it affects the amount of product. The reactant you run out of is called the limiting reagent; Therefore lead (ii) nitrate is the limiting reactant and potassium iodide is in excess. Determine the limiting reactant and calculate the theoretical yield of pbi 2. The procedure of this experiment requires us to use the hol required items and use the. The chemical that is used up is called the limiting reactant while the other reactant is present in excess. The other reactant or reactants are considered to be in excess. If both reactants are present in exactly. The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant.
From www.slideserve.com
PPT Limiting Reactant PowerPoint Presentation, free download ID5054033 Limiting Reactant Hol Lab The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. Determine the limiting reactant and calculate the theoretical yield of pbi 2. In the following experiment trials are done with two different constants to determine the limiting reagents for each reaction are both poles of the. The other reactant or. Limiting Reactant Hol Lab.
From eduinput.com
What is the limiting reactant? Explained with Examples Limiting Reactant Hol Lab To identify the limiting reactant, calculate the number of moles. In the following experiment trials are done with two different constants to determine the limiting reagents for each reaction are both poles of the. Determine the limiting reactant and calculate the theoretical yield of pbi 2. The reactant you run out of is called the limiting reagent; The procedure of. Limiting Reactant Hol Lab.
From www.youtube.com
Limiting Reactant Lab Procedure (Remote) YouTube Limiting Reactant Hol Lab If both reactants are present in exactly. Determine the limiting reactant in the chemical reaction and use measured data to determine how it affects the amount of product. The procedure of this experiment requires us to use the hol required items and use the. Therefore lead (ii) nitrate is the limiting reactant and potassium iodide is in excess. In the. Limiting Reactant Hol Lab.
From www.studocu.com
Limiting reactant lab CHEM Limiting Reactant & Percent Yield Lab In Limiting Reactant Hol Lab Determine the limiting reactant and calculate the theoretical yield of pbi 2. To identify the limiting reactant, calculate the number of moles. The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. The procedure of this experiment requires us to use the hol required items and use the. In the. Limiting Reactant Hol Lab.
From www.youtube.com
Limiting Reactants YouTube Limiting Reactant Hol Lab In the following experiment trials are done with two different constants to determine the limiting reagents for each reaction are both poles of the. Determine the limiting reactant and calculate the theoretical yield of pbi 2. If both reactants are present in exactly. Therefore lead (ii) nitrate is the limiting reactant and potassium iodide is in excess. Determine the limiting. Limiting Reactant Hol Lab.
From www.chegg.com
Solved Limiting Reactant Lab Chem. 101 Limiting Reactant Lab Limiting Reactant Hol Lab The chemical that is used up is called the limiting reactant while the other reactant is present in excess. Determine the limiting reactant in the chemical reaction and use measured data to determine how it affects the amount of product. In the following experiment trials are done with two different constants to determine the limiting reagents for each reaction are. Limiting Reactant Hol Lab.
From www.geeksforgeeks.org
Limiting Reagent Definition, Methods, Solved Examples, and FAQs Limiting Reactant Hol Lab The reactant you run out of is called the limiting reagent; The procedure of this experiment requires us to use the hol required items and use the. The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. The chemical that is used up is called the limiting reactant while the. Limiting Reactant Hol Lab.
From studylib.net
Limiting Reagent Lab Limiting Reactant Hol Lab To identify the limiting reactant, calculate the number of moles. Determine the limiting reactant and calculate the theoretical yield of pbi 2. The procedure of this experiment requires us to use the hol required items and use the. In the following experiment trials are done with two different constants to determine the limiting reagents for each reaction are both poles. Limiting Reactant Hol Lab.
From www.studocu.com
Zn Cl2 Limiting Reactant Lab Limiting Reactant Lab When a reaction Limiting Reactant Hol Lab In the following experiment trials are done with two different constants to determine the limiting reagents for each reaction are both poles of the. The other reactant or reactants are considered to be in excess. The chemical that is used up is called the limiting reactant while the other reactant is present in excess. Therefore lead (ii) nitrate is the. Limiting Reactant Hol Lab.
From www.youtube.com
Limiting Reactant YouTube Limiting Reactant Hol Lab The procedure of this experiment requires us to use the hol required items and use the. The chemical that is used up is called the limiting reactant while the other reactant is present in excess. Therefore lead (ii) nitrate is the limiting reactant and potassium iodide is in excess. If both reactants are present in exactly. In the following experiment. Limiting Reactant Hol Lab.
From www.tes.com
Limiting Reactants GCSE Lesson (SC9c CC9c) Teaching Resources Limiting Reactant Hol Lab The chemical that is used up is called the limiting reactant while the other reactant is present in excess. If both reactants are present in exactly. The other reactant or reactants are considered to be in excess. Determine the limiting reactant in the chemical reaction and use measured data to determine how it affects the amount of product. The procedure. Limiting Reactant Hol Lab.
From www.youtube.com
Determining the Limiting Reactant and Theoretical Yield for a Reaction Limiting Reactant Hol Lab The procedure of this experiment requires us to use the hol required items and use the. Determine the limiting reactant and calculate the theoretical yield of pbi 2. If both reactants are present in exactly. Therefore lead (ii) nitrate is the limiting reactant and potassium iodide is in excess. Determine the limiting reactant in the chemical reaction and use measured. Limiting Reactant Hol Lab.
From studylib.net
Limiting Reactant Practice Limiting Reactant Hol Lab Determine the limiting reactant in the chemical reaction and use measured data to determine how it affects the amount of product. The chemical that is used up is called the limiting reactant while the other reactant is present in excess. Therefore lead (ii) nitrate is the limiting reactant and potassium iodide is in excess. The reactant that is consumed first. Limiting Reactant Hol Lab.
From studylib.net
Experiment 4 Limiting Reactant Limiting Reactant Hol Lab The reactant you run out of is called the limiting reagent; If both reactants are present in exactly. To identify the limiting reactant, calculate the number of moles. Therefore lead (ii) nitrate is the limiting reactant and potassium iodide is in excess. The chemical that is used up is called the limiting reactant while the other reactant is present in. Limiting Reactant Hol Lab.
From studylib.net
LIMITING REAGENT LAB Limiting Reactant Hol Lab If both reactants are present in exactly. Determine the limiting reactant and calculate the theoretical yield of pbi 2. To identify the limiting reactant, calculate the number of moles. The reactant you run out of is called the limiting reagent; In the following experiment trials are done with two different constants to determine the limiting reagents for each reaction are. Limiting Reactant Hol Lab.
From www.studypool.com
SOLUTION Chm 111 lab 5 limiting reagent lab final version Studypool Limiting Reactant Hol Lab Therefore lead (ii) nitrate is the limiting reactant and potassium iodide is in excess. The chemical that is used up is called the limiting reactant while the other reactant is present in excess. Determine the limiting reactant and calculate the theoretical yield of pbi 2. The procedure of this experiment requires us to use the hol required items and use. Limiting Reactant Hol Lab.
From www.youtube.com
Lab Lecture video Lab 6 Limiting and Excess Reactant YouTube Limiting Reactant Hol Lab Determine the limiting reactant in the chemical reaction and use measured data to determine how it affects the amount of product. Determine the limiting reactant and calculate the theoretical yield of pbi 2. The chemical that is used up is called the limiting reactant while the other reactant is present in excess. To identify the limiting reactant, calculate the number. Limiting Reactant Hol Lab.
From www.slideserve.com
PPT Limiting Reactants Lab PowerPoint Presentation, free download Limiting Reactant Hol Lab Therefore lead (ii) nitrate is the limiting reactant and potassium iodide is in excess. The procedure of this experiment requires us to use the hol required items and use the. The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. The other reactant or reactants are considered to be in. Limiting Reactant Hol Lab.
From bradypaukenchemblog.blogspot.com
Limiting Reactant Lab Limiting Reactant Hol Lab If both reactants are present in exactly. To identify the limiting reactant, calculate the number of moles. Determine the limiting reactant in the chemical reaction and use measured data to determine how it affects the amount of product. The chemical that is used up is called the limiting reactant while the other reactant is present in excess. Determine the limiting. Limiting Reactant Hol Lab.
From alaniferscannon.blogspot.com
How to Determine Limiting Reactant Limiting Reactant Hol Lab In the following experiment trials are done with two different constants to determine the limiting reagents for each reaction are both poles of the. The other reactant or reactants are considered to be in excess. The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. The procedure of this experiment. Limiting Reactant Hol Lab.
From www.slideserve.com
PPT Laboratory 08 LIMITING REACTANT LAB PowerPoint Presentation, free Limiting Reactant Hol Lab If both reactants are present in exactly. The procedure of this experiment requires us to use the hol required items and use the. The reactant you run out of is called the limiting reagent; The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. To identify the limiting reactant, calculate. Limiting Reactant Hol Lab.
From worksheetzone.org
Limiting Reactant Example Worksheet Limiting Reactant Hol Lab In the following experiment trials are done with two different constants to determine the limiting reagents for each reaction are both poles of the. The other reactant or reactants are considered to be in excess. Determine the limiting reactant in the chemical reaction and use measured data to determine how it affects the amount of product. The procedure of this. Limiting Reactant Hol Lab.
From www.slideserve.com
PPT Laboratory 08 LIMITING REACTANT LAB PowerPoint Presentation, free Limiting Reactant Hol Lab Determine the limiting reactant and calculate the theoretical yield of pbi 2. If both reactants are present in exactly. In the following experiment trials are done with two different constants to determine the limiting reagents for each reaction are both poles of the. To identify the limiting reactant, calculate the number of moles. The chemical that is used up is. Limiting Reactant Hol Lab.
From about.dataclassroom.com
Limiting Reactants Lab — DataClassroom Limiting Reactant Hol Lab Therefore lead (ii) nitrate is the limiting reactant and potassium iodide is in excess. The procedure of this experiment requires us to use the hol required items and use the. Determine the limiting reactant in the chemical reaction and use measured data to determine how it affects the amount of product. In the following experiment trials are done with two. Limiting Reactant Hol Lab.
From www.slideserve.com
PPT Laboratory 08 LIMITING REACTANT LAB PowerPoint Presentation, free Limiting Reactant Hol Lab Therefore lead (ii) nitrate is the limiting reactant and potassium iodide is in excess. The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. Determine the limiting reactant in the chemical reaction and use measured data to determine how it affects the amount of product. To identify the limiting reactant,. Limiting Reactant Hol Lab.
From www.studypool.com
SOLUTION Stoichiometry And Limiting Reactant Lab Studypool Limiting Reactant Hol Lab To identify the limiting reactant, calculate the number of moles. Determine the limiting reactant in the chemical reaction and use measured data to determine how it affects the amount of product. The chemical that is used up is called the limiting reactant while the other reactant is present in excess. The reactant you run out of is called the limiting. Limiting Reactant Hol Lab.
From chemistry.analia-sanchez.net
Limiting Reactant Notes Chemistry Classes / Ronald Reagan S.H.S. Limiting Reactant Hol Lab The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. To identify the limiting reactant, calculate the number of moles. The other reactant or reactants are considered to be in excess. If both reactants are present in exactly. Therefore lead (ii) nitrate is the limiting reactant and potassium iodide is. Limiting Reactant Hol Lab.
From www.slideserve.com
PPT Limiting Reactants (Reagents) PowerPoint Presentation, free Limiting Reactant Hol Lab In the following experiment trials are done with two different constants to determine the limiting reagents for each reaction are both poles of the. The chemical that is used up is called the limiting reactant while the other reactant is present in excess. If both reactants are present in exactly. The other reactant or reactants are considered to be in. Limiting Reactant Hol Lab.
From about.dataclassroom.com
Limiting Reactants Lab — DataClassroom Limiting Reactant Hol Lab The procedure of this experiment requires us to use the hol required items and use the. Determine the limiting reactant and calculate the theoretical yield of pbi 2. Therefore lead (ii) nitrate is the limiting reactant and potassium iodide is in excess. To identify the limiting reactant, calculate the number of moles. Determine the limiting reactant in the chemical reaction. Limiting Reactant Hol Lab.
From lessonlistelois.z13.web.core.windows.net
Limiting Reactant Lab Answers Limiting Reactant Hol Lab Determine the limiting reactant in the chemical reaction and use measured data to determine how it affects the amount of product. The other reactant or reactants are considered to be in excess. The chemical that is used up is called the limiting reactant while the other reactant is present in excess. To identify the limiting reactant, calculate the number of. Limiting Reactant Hol Lab.
From www.scribd.com
Limiting Reactant Practice Key PDF Limiting Reactant Hol Lab The chemical that is used up is called the limiting reactant while the other reactant is present in excess. The reactant you run out of is called the limiting reagent; The other reactant or reactants are considered to be in excess. Determine the limiting reactant in the chemical reaction and use measured data to determine how it affects the amount. Limiting Reactant Hol Lab.
From quizzlibvasbinder.z21.web.core.windows.net
How To Determine Limiting And Excess Reactant Limiting Reactant Hol Lab The reactant you run out of is called the limiting reagent; Therefore lead (ii) nitrate is the limiting reactant and potassium iodide is in excess. To identify the limiting reactant, calculate the number of moles. Determine the limiting reactant and calculate the theoretical yield of pbi 2. In the following experiment trials are done with two different constants to determine. Limiting Reactant Hol Lab.
From www.studypool.com
SOLUTION Chm 111 lab 5 limiting reagent lab final version Studypool Limiting Reactant Hol Lab The reactant you run out of is called the limiting reagent; The other reactant or reactants are considered to be in excess. Therefore lead (ii) nitrate is the limiting reactant and potassium iodide is in excess. In the following experiment trials are done with two different constants to determine the limiting reagents for each reaction are both poles of the.. Limiting Reactant Hol Lab.
From www.slideserve.com
PPT Laboratory 08 LIMITING REACTANT LAB PowerPoint Presentation, free Limiting Reactant Hol Lab Determine the limiting reactant in the chemical reaction and use measured data to determine how it affects the amount of product. The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. To identify the limiting reactant, calculate the number of moles. The chemical that is used up is called the. Limiting Reactant Hol Lab.
From chemistry101efhs.weebly.com
Limiting Reactants Chemistry 101 Limiting Reactant Hol Lab The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. To identify the limiting reactant, calculate the number of moles. In the following experiment trials are done with two different constants to determine the limiting reagents for each reaction are both poles of the. The procedure of this experiment requires. Limiting Reactant Hol Lab.