What Is Chlorine An Ion Charge . 93 rows ionic charge: Uncombined elements have an oxidation state of 0. It forms strong ionic bonds with metal ions. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. Neutral chlorine atom on left has 17 protons and 17 electrons. 93 rows this table shows the most common charges for atoms of the chemical elements. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring. Using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for chlorine. You can use this table to predict whether an atom can bond with another atom. There are two chloride ions in the formula. It is defined as being the charge that an atom would have if all bonds were ionic.
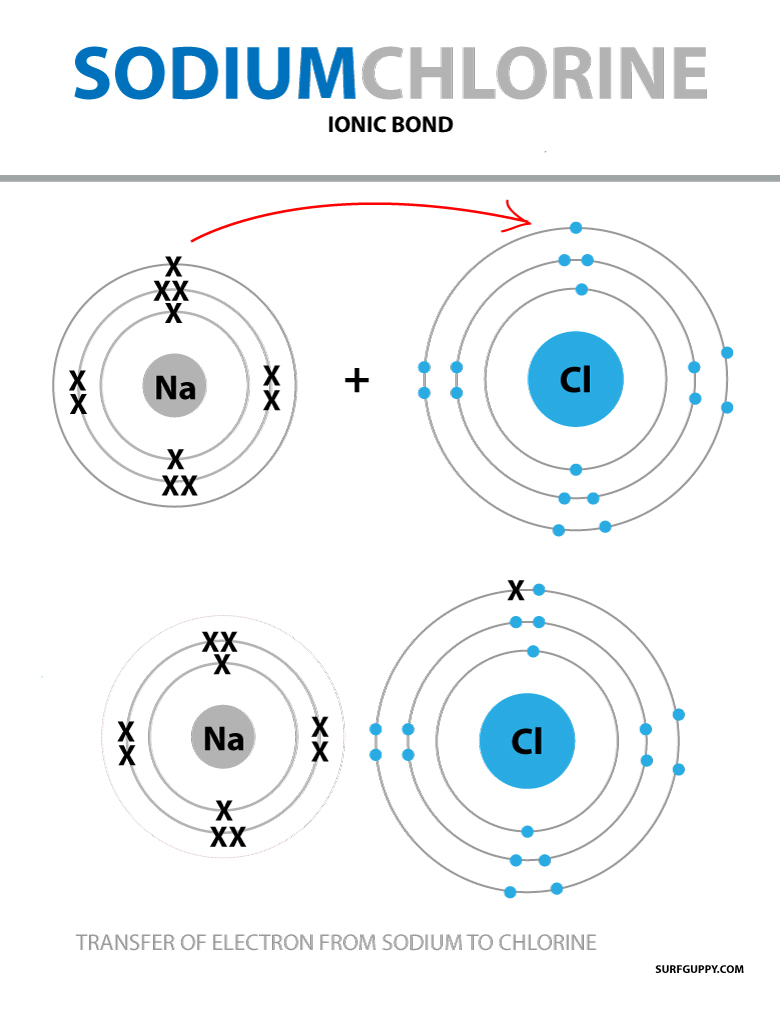
from surfguppy.com
You can use this table to predict whether an atom can bond with another atom. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is. 93 rows ionic charge: 93 rows this table shows the most common charges for atoms of the chemical elements. It forms strong ionic bonds with metal ions. Using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for chlorine. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. Neutral chlorine atom on left has 17 protons and 17 electrons. It is defined as being the charge that an atom would have if all bonds were ionic.
What is Ionic Bond Surfguppy Chemistry made easy visual learning
What Is Chlorine An Ion Charge You can use this table to predict whether an atom can bond with another atom. Neutral chlorine atom on left has 17 protons and 17 electrons. Uncombined elements have an oxidation state of 0. It is defined as being the charge that an atom would have if all bonds were ionic. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is. 93 rows ionic charge: You can use this table to predict whether an atom can bond with another atom. Using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for chlorine. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring. It forms strong ionic bonds with metal ions. 93 rows this table shows the most common charges for atoms of the chemical elements. There are two chloride ions in the formula.
From www.sciencenewsforstudents.org
Explainer Ions and radicals in our world Science News for Students What Is Chlorine An Ion Charge It is defined as being the charge that an atom would have if all bonds were ionic. Neutral chlorine atom on left has 17 protons and 17 electrons. Uncombined elements have an oxidation state of 0. 93 rows this table shows the most common charges for atoms of the chemical elements. Using a simple, general trend for the ionic charge. What Is Chlorine An Ion Charge.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts What Is Chlorine An Ion Charge 93 rows ionic charge: Using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for chlorine. There are two chloride ions in the formula. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. You can use this table to. What Is Chlorine An Ion Charge.
From sciencenotes.org
Cations and Anions Definitions, Examples, and Differences What Is Chlorine An Ion Charge Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring. It is defined as being the charge that an atom would have if all bonds were ionic. It forms strong ionic bonds with metal ions. 93 rows. What Is Chlorine An Ion Charge.
From www.chemistrystudent.com
Ionic Bonding (ALevel) ChemistryStudent What Is Chlorine An Ion Charge When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is. It is defined as being the charge that an atom would have if all bonds were ionic. 93 rows ionic charge: Neutral chlorine atom on left has 17 protons and 17 electrons. You can use this table to predict whether an. What Is Chlorine An Ion Charge.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table What Is Chlorine An Ion Charge Using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for chlorine. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is. 93 rows this table shows the most common charges for atoms of the chemical elements. There. What Is Chlorine An Ion Charge.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine What Is Chlorine An Ion Charge Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring. 93 rows this table shows the most common charges for atoms of the chemical elements. 93 rows ionic charge: You can use this table to predict whether an atom can bond with another atom. Although chlorine as an element is a diatomic molecule, cl. What Is Chlorine An Ion Charge.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons What Is Chlorine An Ion Charge 93 rows this table shows the most common charges for atoms of the chemical elements. There are two chloride ions in the formula. Neutral chlorine atom on left has 17 protons and 17 electrons. Uncombined elements have an oxidation state of 0. It forms strong ionic bonds with metal ions. 93 rows ionic charge: When the atom loses or gains. What Is Chlorine An Ion Charge.
From surfguppy.com
What is Ionic Bond Surfguppy Chemistry made easy visual learning What Is Chlorine An Ion Charge Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. Using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for chlorine. It forms strong ionic bonds with metal ions. It is defined as being the charge that an atom. What Is Chlorine An Ion Charge.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram What Is Chlorine An Ion Charge 93 rows this table shows the most common charges for atoms of the chemical elements. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is. There are two chloride ions in the formula. It is defined as being the charge that an atom would have if all bonds were ionic. Like. What Is Chlorine An Ion Charge.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of What Is Chlorine An Ion Charge It is defined as being the charge that an atom would have if all bonds were ionic. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. You can use this table to predict. What Is Chlorine An Ion Charge.
From www.shalom-education.com
Reactions of Halogens GCSE Chemistry Revision What Is Chlorine An Ion Charge Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. There are two chloride ions in the formula. It is defined as being the charge that an atom would have if all bonds were ionic. It forms strong ionic bonds with metal ions. Like fluorine and the other members of the halogen family,. What Is Chlorine An Ion Charge.
From www.sliderbase.com
Ions What Is Chlorine An Ion Charge It is defined as being the charge that an atom would have if all bonds were ionic. 93 rows this table shows the most common charges for atoms of the chemical elements. Using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for chlorine. It forms strong. What Is Chlorine An Ion Charge.
From www.animalia-life.club
Electron Configuration For Chlorine What Is Chlorine An Ion Charge It forms strong ionic bonds with metal ions. Using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for chlorine. 93 rows this table shows the most common charges for atoms of the chemical elements. Uncombined elements have an oxidation state of 0. You can use this. What Is Chlorine An Ion Charge.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions What Is Chlorine An Ion Charge Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring. There are two chloride ions in the formula. It forms strong ionic bonds with metal ions. 93 rows ionic charge: Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. 93 rows this table shows the most. What Is Chlorine An Ion Charge.
From science4fun.info
Chlorine Element (Properties, Uses, and Facts) Science4Fun What Is Chlorine An Ion Charge 93 rows ionic charge: Uncombined elements have an oxidation state of 0. It is defined as being the charge that an atom would have if all bonds were ionic. There are two chloride ions in the formula. Using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge. What Is Chlorine An Ion Charge.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 What Is Chlorine An Ion Charge 93 rows ionic charge: You can use this table to predict whether an atom can bond with another atom. Neutral chlorine atom on left has 17 protons and 17 electrons. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring. There are two chloride ions in the formula. When the atom loses or gains. What Is Chlorine An Ion Charge.
From www.askiitians.com
Chlorine Study Material for IIT JEE askIITians What Is Chlorine An Ion Charge You can use this table to predict whether an atom can bond with another atom. There are two chloride ions in the formula. 93 rows this table shows the most common charges for atoms of the chemical elements. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. Like fluorine and the other. What Is Chlorine An Ion Charge.
From delorescgough.blob.core.windows.net
Chlorine Electron Charge at delorescgough blog What Is Chlorine An Ion Charge Neutral chlorine atom on left has 17 protons and 17 electrons. 93 rows ionic charge: Uncombined elements have an oxidation state of 0. There are two chloride ions in the formula. Using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for chlorine. Although chlorine as an. What Is Chlorine An Ion Charge.
From www.youtube.com
How to Draw the Lewis Structure for ClO2 (Chlorine dioxide) YouTube What Is Chlorine An Ion Charge 93 rows this table shows the most common charges for atoms of the chemical elements. Using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for chlorine. Neutral chlorine atom on left has 17 protons and 17 electrons. Although chlorine as an element is a diatomic molecule,. What Is Chlorine An Ion Charge.
From www.youtube.com
How to find Protons & Electrons for the Chloride ion (Cl) YouTube What Is Chlorine An Ion Charge Neutral chlorine atom on left has 17 protons and 17 electrons. You can use this table to predict whether an atom can bond with another atom. It is defined as being the charge that an atom would have if all bonds were ionic. There are two chloride ions in the formula. Like fluorine and the other members of the halogen. What Is Chlorine An Ion Charge.
From slideplayer.com
What are ionic compounds and how do they form? ppt download What Is Chlorine An Ion Charge Uncombined elements have an oxidation state of 0. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring. 93 rows this table shows the most common charges for atoms of the chemical elements. It is defined as being the charge that an atom would have if all bonds were ionic. It forms strong ionic. What Is Chlorine An Ion Charge.
From www.youtube.com
How to Draw the Lewis Dot Structure for Cl (Chloride ion) YouTube What Is Chlorine An Ion Charge You can use this table to predict whether an atom can bond with another atom. Neutral chlorine atom on left has 17 protons and 17 electrons. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. It. What Is Chlorine An Ion Charge.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image What Is Chlorine An Ion Charge When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is. 93 rows this table shows the most common charges for atoms of the chemical elements. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring. Uncombined elements have an oxidation state of 0. It is. What Is Chlorine An Ion Charge.
From www.numerade.com
SOLVED The Lewis dot symbol for the chloride ion is The Lewis dot What Is Chlorine An Ion Charge It is defined as being the charge that an atom would have if all bonds were ionic. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring. Neutral chlorine atom on left has 17 protons and 17 electrons. 93 rows this table shows the most common charges for atoms of the chemical elements. There. What Is Chlorine An Ion Charge.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID4192871 What Is Chlorine An Ion Charge 93 rows this table shows the most common charges for atoms of the chemical elements. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is. Using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for chlorine. 93. What Is Chlorine An Ion Charge.
From www.alamy.com
Chlorine electron configuration. Illustration of the atomic structure What Is Chlorine An Ion Charge When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is. 93 rows ionic charge: Uncombined elements have an oxidation state of 0. 93 rows this table shows the most common charges for atoms of the chemical elements. Neutral chlorine atom on left has 17 protons and 17 electrons. There are two. What Is Chlorine An Ion Charge.
From 2012books.lardbucket.org
Ions What Is Chlorine An Ion Charge 93 rows this table shows the most common charges for atoms of the chemical elements. Neutral chlorine atom on left has 17 protons and 17 electrons. 93 rows ionic charge: You can use this table to predict whether an atom can bond with another atom. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature,. What Is Chlorine An Ion Charge.
From www.expii.com
Ions — Definition & Overview Expii What Is Chlorine An Ion Charge You can use this table to predict whether an atom can bond with another atom. Neutral chlorine atom on left has 17 protons and 17 electrons. There are two chloride ions in the formula. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring. It forms strong ionic bonds with metal ions. 93 rows. What Is Chlorine An Ion Charge.
From www.youtube.com
Chlorine Electron Configuration YouTube What Is Chlorine An Ion Charge 93 rows this table shows the most common charges for atoms of the chemical elements. It is defined as being the charge that an atom would have if all bonds were ionic. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. Like fluorine and the other members of the halogen family, chlorine. What Is Chlorine An Ion Charge.
From philschatz.com
Chemical Bonds · Anatomy and Physiology What Is Chlorine An Ion Charge You can use this table to predict whether an atom can bond with another atom. Using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for chlorine. Uncombined elements have an oxidation state of 0. When the atom loses or gains one or more electrons, the electric. What Is Chlorine An Ion Charge.
From en.wikipedia.org
Ionic bonding Wikipedia What Is Chlorine An Ion Charge There are two chloride ions in the formula. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring. 93 rows ionic charge: It forms strong ionic bonds with metal ions. Uncombined elements have an oxidation state of 0. It is defined as being the charge that an atom would have if all bonds were. What Is Chlorine An Ion Charge.
From slidesharenow.blogspot.com
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare What Is Chlorine An Ion Charge Uncombined elements have an oxidation state of 0. Using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for chlorine. You can use this table to predict whether an atom can bond with another atom. 93 rows ionic charge: It forms strong ionic bonds with metal ions.. What Is Chlorine An Ion Charge.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and What Is Chlorine An Ion Charge Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring. 93 rows this table shows the most common charges for atoms of the chemical elements. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. Using a simple, general trend for the ionic charge for elements on. What Is Chlorine An Ion Charge.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons What Is Chlorine An Ion Charge Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. Uncombined elements have an oxidation state of 0. Using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for chlorine. You can use this table to predict whether an atom. What Is Chlorine An Ion Charge.
From www.vrogue.co
Orbital Diagram For Chlorine vrogue.co What Is Chlorine An Ion Charge It is defined as being the charge that an atom would have if all bonds were ionic. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is. You can use this table to predict whether an atom can bond with another atom. Neutral chlorine atom on left has 17 protons and. What Is Chlorine An Ion Charge.