Validation Of Laboratory Results . Validation procedures are essential in medical laboratory testing to ensure the accuracy and reliability of. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. A method validation provides proof that a method is suited for its intended use and that it fulfills the necessary quality requirements. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be. Validation and total quality management elements with close attention to the work flow of the laboratory ensure that accurate and precise. A key component of the quality.
from www.euformatics.com
The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be. A key component of the quality. To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. Validation and total quality management elements with close attention to the work flow of the laboratory ensure that accurate and precise. Validation procedures are essential in medical laboratory testing to ensure the accuracy and reliability of. A method validation provides proof that a method is suited for its intended use and that it fulfills the necessary quality requirements.
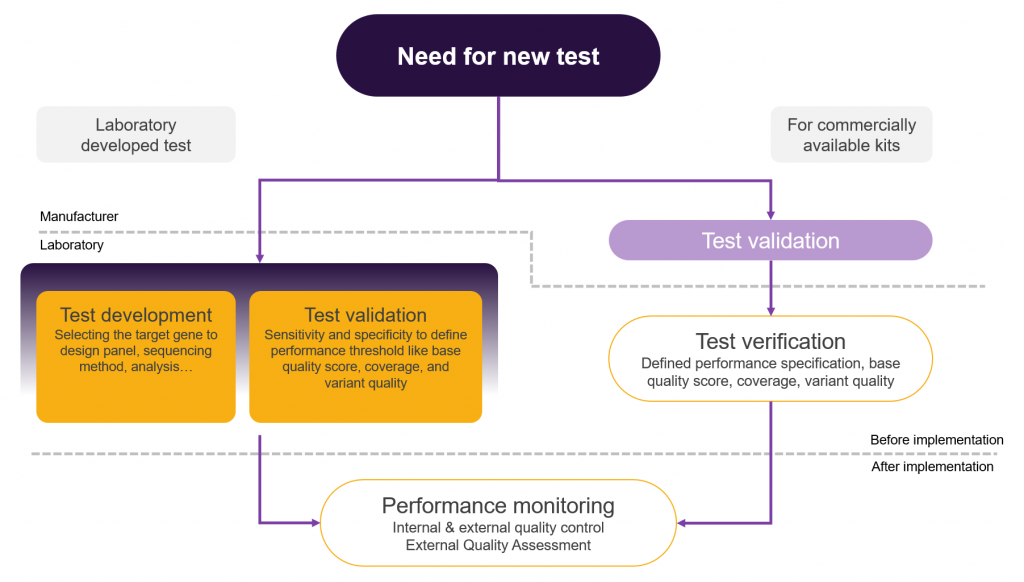
Validation of NGS clinical tests Euformatics
Validation Of Laboratory Results The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be. A key component of the quality. Validation and total quality management elements with close attention to the work flow of the laboratory ensure that accurate and precise. A method validation provides proof that a method is suited for its intended use and that it fulfills the necessary quality requirements. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be. Validation procedures are essential in medical laboratory testing to ensure the accuracy and reliability of. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable.
From www.europeanlabservices.eu
Validation in Pharma European Lab Services ELS Validation Of Laboratory Results Validation and total quality management elements with close attention to the work flow of the laboratory ensure that accurate and precise. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. Validation procedures are essential in medical laboratory testing to ensure the accuracy and reliability of. A. Validation Of Laboratory Results.
From www.researchgate.net
(PDF) Validation of Clinical Laboratory Results Discussion of Validation Of Laboratory Results The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be. Validation and total quality management elements with close attention to the work flow of the laboratory ensure that accurate and precise. To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. This review. Validation Of Laboratory Results.
From www.prolisphere.com
Why is validation important for a laboratory analyzer Prolis Validation Of Laboratory Results A method validation provides proof that a method is suited for its intended use and that it fulfills the necessary quality requirements. Validation procedures are essential in medical laboratory testing to ensure the accuracy and reliability of. Validation and total quality management elements with close attention to the work flow of the laboratory ensure that accurate and precise. A key. Validation Of Laboratory Results.
From www.scribd.com
validation of laboratory results PDF Medical Laboratory Health Care Validation Of Laboratory Results The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be. A method validation provides proof that a method is suited for its intended use and that it fulfills the necessary quality requirements. Validation and total quality management elements with close attention to the work flow of the. Validation Of Laboratory Results.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Validation Of Laboratory Results A key component of the quality. A method validation provides proof that a method is suited for its intended use and that it fulfills the necessary quality requirements. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. Validation and total quality management elements with close attention. Validation Of Laboratory Results.
From www.prolisphere.com
Why is validation important for a laboratory analyzer Prolis Validation Of Laboratory Results This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. Validation procedures are essential in medical laboratory testing to ensure the accuracy and reliability of. To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. Validation and total quality management elements with close. Validation Of Laboratory Results.
From www.degruyter.com
Validation and verification of examination procedures in medical Validation Of Laboratory Results This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. A method validation provides proof that a method is suited for its intended use and that it fulfills the necessary quality requirements. Validation and total quality management elements with close attention to the work flow of the. Validation Of Laboratory Results.
From www.researchgate.net
Results of the laboratory validation experiment. The uncorrected Validation Of Laboratory Results To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. Validation procedures are essential in medical laboratory testing to ensure the accuracy and reliability of. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. Validation and total quality management elements with close. Validation Of Laboratory Results.
From dokumen.tips
(PDF) Laboratory validation of a clinical metagenomic sequencing Validation Of Laboratory Results Validation and total quality management elements with close attention to the work flow of the laboratory ensure that accurate and precise. A key component of the quality. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. To ensure correct diagnosis and treatment, clinical laboratory testing must. Validation Of Laboratory Results.
From www.euformatics.com
Validation of NGS clinical tests Euformatics Validation Of Laboratory Results This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. Validation and total quality management elements with close attention to the work flow of the laboratory ensure that accurate and precise. A key component of the quality. Validation procedures are essential in medical laboratory testing to ensure. Validation Of Laboratory Results.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Validation Of Laboratory Results Validation procedures are essential in medical laboratory testing to ensure the accuracy and reliability of. To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. A method validation provides proof that a method is suited for its intended use and that it fulfills the necessary quality requirements. A key component of the quality. Validation and total. Validation Of Laboratory Results.
From www.researchgate.net
Acceptance criteria and results of the method validation parameters Validation Of Laboratory Results This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be. Validation and total quality management elements with close attention to the work flow of. Validation Of Laboratory Results.
From mavink.com
Laboratory Validation Template Validation Of Laboratory Results A method validation provides proof that a method is suited for its intended use and that it fulfills the necessary quality requirements. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. A key component of the quality. Validation and total quality management elements with close attention. Validation Of Laboratory Results.
From www.researchgate.net
Procedures for Validation of Diagnostic Methods in Clinical Laboratory Validation Of Laboratory Results This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. A key component of the quality. Validation and total quality management elements with close attention to the work flow of the laboratory ensure that accurate and precise. A method validation provides proof that a method is suited. Validation Of Laboratory Results.
From www.researchgate.net
Workflow of the prevalidation experiments. The number of laboratories Validation Of Laboratory Results A key component of the quality. Validation and total quality management elements with close attention to the work flow of the laboratory ensure that accurate and precise. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be. This review summarizes the current literature on the topic, focusing. Validation Of Laboratory Results.
From foracare.com
Clinical Validation ForaCare Validation Of Laboratory Results A key component of the quality. Validation procedures are essential in medical laboratory testing to ensure the accuracy and reliability of. To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. A method validation provides proof that a method is suited for its intended use and that it fulfills the necessary quality requirements. Validation and total. Validation Of Laboratory Results.
From www.youtube.com
How to Validate and Verify the Accuracy of your Clinical Laboratory Validation Of Laboratory Results The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. A key component of the quality. Validation and total quality management elements with close. Validation Of Laboratory Results.
From www.chromatographyonline.com
GAMP Good Practice Guide for Validation of Laboratory Computerized Validation Of Laboratory Results A method validation provides proof that a method is suited for its intended use and that it fulfills the necessary quality requirements. A key component of the quality. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. Validation and total quality management elements with close attention. Validation Of Laboratory Results.
From rjqualityconsulting.com
ISO 17025 Method Validation Ensuring Laboratory Competence and Validation Of Laboratory Results The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be. Validation and total quality management elements with close attention to the work flow of the laboratory ensure that accurate and precise. Validation procedures are essential in medical laboratory testing to ensure the accuracy and reliability of. A. Validation Of Laboratory Results.
From www.researchgate.net
Template of a validation plan. Download Scientific Diagram Validation Of Laboratory Results To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. Validation procedures are essential in medical laboratory testing to ensure the accuracy and reliability of. A method validation provides proof that a method is suited for its intended use and that it fulfills the necessary quality requirements. A key component of the quality. This review summarizes. Validation Of Laboratory Results.
From www.genebygene.com
Clinical Validation Gene by Gene Laboratory Services Validation Of Laboratory Results Validation procedures are essential in medical laboratory testing to ensure the accuracy and reliability of. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. A key component of the quality. A method validation provides proof that a method is suited for its intended use and that. Validation Of Laboratory Results.
From www.aapc.com
Clinical Validation The Next Big Challenge in Inpatient Coding AAPC Validation Of Laboratory Results This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be.. Validation Of Laboratory Results.
From studylib.net
Laboratory Method Validation For any method that will be used for Validation Of Laboratory Results A method validation provides proof that a method is suited for its intended use and that it fulfills the necessary quality requirements. A key component of the quality. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be. Validation procedures are essential in medical laboratory testing to. Validation Of Laboratory Results.
From www.researchgate.net
Laboratory verification and clinical validation of a T cell assay for Validation Of Laboratory Results The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. Validation procedures are essential in medical laboratory testing to ensure the accuracy and reliability. Validation Of Laboratory Results.
From www.researchgate.net
Template of a validation certificate. Download Scientific Diagram Validation Of Laboratory Results To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. A method validation provides proof that a method is suited for its intended use and that it fulfills the necessary quality requirements. Validation and total quality management elements with close attention to the work flow of the laboratory ensure that accurate and precise. A key component. Validation Of Laboratory Results.
From exyqurouh.blob.core.windows.net
Sample Lab Results Report at Reid blog Validation Of Laboratory Results Validation procedures are essential in medical laboratory testing to ensure the accuracy and reliability of. Validation and total quality management elements with close attention to the work flow of the laboratory ensure that accurate and precise. To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. The purpose of this toolkit is to assist laboratories in. Validation Of Laboratory Results.
From www.researchgate.net
Laboratory test results of the patients Download Scientific Diagram Validation Of Laboratory Results Validation and total quality management elements with close attention to the work flow of the laboratory ensure that accurate and precise. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be. To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. A method. Validation Of Laboratory Results.
From www.parkview.com
How to interpret your lab test results Parkview Health Validation Of Laboratory Results This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. Validation and total quality management elements with close attention to the work flow of the laboratory ensure that accurate and precise. To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. A method. Validation Of Laboratory Results.
From cptclabs.com
Reliable Method Validation FDA Compliant CPT Labs Validation Of Laboratory Results A key component of the quality. Validation and total quality management elements with close attention to the work flow of the laboratory ensure that accurate and precise. To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification,. Validation Of Laboratory Results.
From clsjournal.ascls.org
FDAapproved, Nonwaived Laboratory Tests Method Validation Validation Of Laboratory Results To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. Validation and total quality management elements with close attention to the work flow of the laboratory ensure that accurate and precise. A key component of the quality. A method validation provides proof that a method is suited for its intended use and that it fulfills the. Validation Of Laboratory Results.
From www.researchgate.net
Results of the 1991 laboratory validation experiment comparing the Validation Of Laboratory Results A key component of the quality. A method validation provides proof that a method is suited for its intended use and that it fulfills the necessary quality requirements. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be. Validation procedures are essential in medical laboratory testing to. Validation Of Laboratory Results.
From biotechserv601227982.wordpress.com
The Importance of Lab Equipment Validation Biotechnical Services Validation Of Laboratory Results Validation procedures are essential in medical laboratory testing to ensure the accuracy and reliability of. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should. Validation Of Laboratory Results.
From www.scribd.com
Method Validation Report Template 1 Experiment Accuracy And Precision Validation Of Laboratory Results The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be. A method validation provides proof that a method is suited for its intended use and that it fulfills the necessary quality requirements. Validation and total quality management elements with close attention to the work flow of the. Validation Of Laboratory Results.
From www.researchgate.net
selection for clinical laboratory test method validation in Validation Of Laboratory Results A method validation provides proof that a method is suited for its intended use and that it fulfills the necessary quality requirements. Validation and total quality management elements with close attention to the work flow of the laboratory ensure that accurate and precise. Validation procedures are essential in medical laboratory testing to ensure the accuracy and reliability of. This review. Validation Of Laboratory Results.
From www.researchgate.net
(PDF) Validation of Clinical Laboratory Results Discussion of Validation Of Laboratory Results The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. A key component of the quality. Validation procedures are essential in medical laboratory testing. Validation Of Laboratory Results.