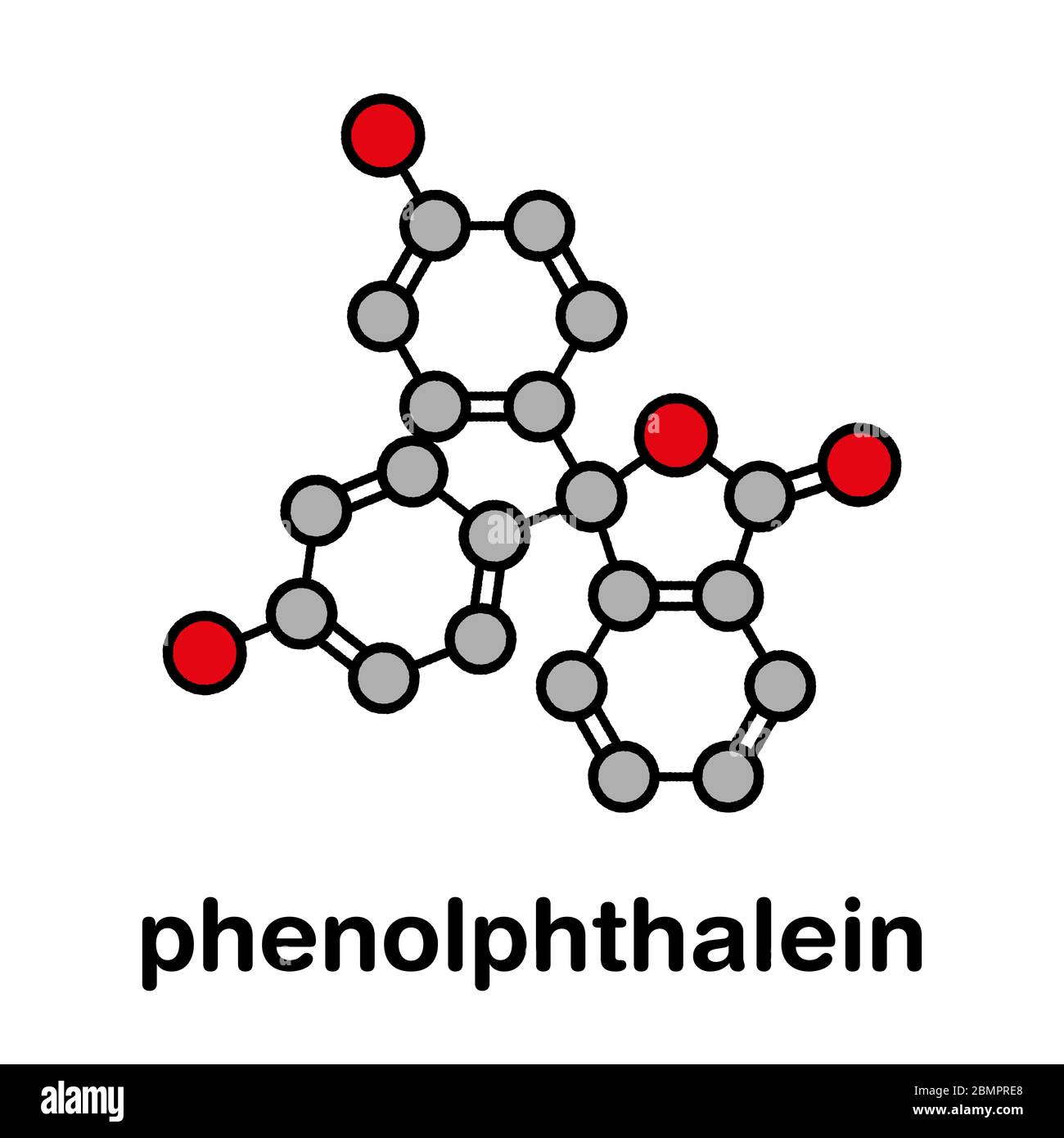
Phenolphthalein Indicators . Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Adding extra hydrogen ions shifts. Learn how phenolphthalein works as a weak acid indicator for titrations, and how its color changes with ph. Find out its pk ind value, ph. As an indicator of a solution’s ph, phenolphthalein is colourless below ph 8.5 and attains a pink to deep red hue above ph 9.0. Find out how to select, use and calculate the acid dissociation constants of indicators. Learn about phenolphthalein, a ph indicator that turns pink in alkaline solutions and colorless in acidic and strongly alkaline solutions. In this case, the weak acid is colourless and its ion is bright pink. See the structure, equilibrium, and ph range of phenolphthalein and other indicators.
from
Learn how phenolphthalein works as a weak acid indicator for titrations, and how its color changes with ph. Find out its pk ind value, ph. See the structure, equilibrium, and ph range of phenolphthalein and other indicators. As an indicator of a solution’s ph, phenolphthalein is colourless below ph 8.5 and attains a pink to deep red hue above ph 9.0. Adding extra hydrogen ions shifts. In this case, the weak acid is colourless and its ion is bright pink. Find out how to select, use and calculate the acid dissociation constants of indicators. Learn about phenolphthalein, a ph indicator that turns pink in alkaline solutions and colorless in acidic and strongly alkaline solutions. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid.
Phenolphthalein Indicators See the structure, equilibrium, and ph range of phenolphthalein and other indicators. Find out how to select, use and calculate the acid dissociation constants of indicators. Learn how phenolphthalein works as a weak acid indicator for titrations, and how its color changes with ph. Adding extra hydrogen ions shifts. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. See the structure, equilibrium, and ph range of phenolphthalein and other indicators. In this case, the weak acid is colourless and its ion is bright pink. Find out its pk ind value, ph. As an indicator of a solution’s ph, phenolphthalein is colourless below ph 8.5 and attains a pink to deep red hue above ph 9.0. Learn about phenolphthalein, a ph indicator that turns pink in alkaline solutions and colorless in acidic and strongly alkaline solutions.
From
Phenolphthalein Indicators Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Learn about phenolphthalein, a ph indicator that turns pink in alkaline solutions and colorless in acidic and strongly alkaline solutions. Find out how to select, use and calculate the acid dissociation constants of indicators. In this case, the weak acid is colourless and its ion is bright. Phenolphthalein Indicators.
From
Phenolphthalein Indicators See the structure, equilibrium, and ph range of phenolphthalein and other indicators. Adding extra hydrogen ions shifts. Learn about phenolphthalein, a ph indicator that turns pink in alkaline solutions and colorless in acidic and strongly alkaline solutions. In this case, the weak acid is colourless and its ion is bright pink. Find out its pk ind value, ph. Phenolphthalein is. Phenolphthalein Indicators.
From
Phenolphthalein Indicators See the structure, equilibrium, and ph range of phenolphthalein and other indicators. Learn how phenolphthalein works as a weak acid indicator for titrations, and how its color changes with ph. Find out its pk ind value, ph. Adding extra hydrogen ions shifts. Learn about phenolphthalein, a ph indicator that turns pink in alkaline solutions and colorless in acidic and strongly. Phenolphthalein Indicators.
From
Phenolphthalein Indicators Learn about phenolphthalein, a ph indicator that turns pink in alkaline solutions and colorless in acidic and strongly alkaline solutions. Adding extra hydrogen ions shifts. See the structure, equilibrium, and ph range of phenolphthalein and other indicators. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Find out its pk ind value, ph. In this case,. Phenolphthalein Indicators.
From
Phenolphthalein Indicators Adding extra hydrogen ions shifts. See the structure, equilibrium, and ph range of phenolphthalein and other indicators. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid is colourless and its ion is bright pink. Find out how to select, use and calculate the acid dissociation constants of indicators. Find out. Phenolphthalein Indicators.
From
Phenolphthalein Indicators As an indicator of a solution’s ph, phenolphthalein is colourless below ph 8.5 and attains a pink to deep red hue above ph 9.0. Find out its pk ind value, ph. In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Learn how. Phenolphthalein Indicators.
From
Phenolphthalein Indicators Find out how to select, use and calculate the acid dissociation constants of indicators. See the structure, equilibrium, and ph range of phenolphthalein and other indicators. As an indicator of a solution’s ph, phenolphthalein is colourless below ph 8.5 and attains a pink to deep red hue above ph 9.0. Find out its pk ind value, ph. Learn about phenolphthalein,. Phenolphthalein Indicators.
From
Phenolphthalein Indicators Find out its pk ind value, ph. As an indicator of a solution’s ph, phenolphthalein is colourless below ph 8.5 and attains a pink to deep red hue above ph 9.0. See the structure, equilibrium, and ph range of phenolphthalein and other indicators. Find out how to select, use and calculate the acid dissociation constants of indicators. Phenolphthalein is another. Phenolphthalein Indicators.
From www.sciencephoto.com
Phenolphthalein Indicator Stock Image C030/7311 Science Photo Library Phenolphthalein Indicators In this case, the weak acid is colourless and its ion is bright pink. Adding extra hydrogen ions shifts. Learn how phenolphthalein works as a weak acid indicator for titrations, and how its color changes with ph. Find out its pk ind value, ph. As an indicator of a solution’s ph, phenolphthalein is colourless below ph 8.5 and attains a. Phenolphthalein Indicators.
From
Phenolphthalein Indicators In this case, the weak acid is colourless and its ion is bright pink. As an indicator of a solution’s ph, phenolphthalein is colourless below ph 8.5 and attains a pink to deep red hue above ph 9.0. See the structure, equilibrium, and ph range of phenolphthalein and other indicators. Find out its pk ind value, ph. Adding extra hydrogen. Phenolphthalein Indicators.
From
Phenolphthalein Indicators Find out its pk ind value, ph. Learn about phenolphthalein, a ph indicator that turns pink in alkaline solutions and colorless in acidic and strongly alkaline solutions. Adding extra hydrogen ions shifts. Find out how to select, use and calculate the acid dissociation constants of indicators. See the structure, equilibrium, and ph range of phenolphthalein and other indicators. Phenolphthalein is. Phenolphthalein Indicators.
From
Phenolphthalein Indicators Find out how to select, use and calculate the acid dissociation constants of indicators. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Learn about phenolphthalein, a ph indicator that turns pink in alkaline solutions and colorless in acidic and strongly alkaline solutions. In this case, the weak acid is colourless and its ion is bright. Phenolphthalein Indicators.
From fphoto.photoshelter.com
science chemistry titration phenolphthalein Fundamental Photographs The Art of Science Phenolphthalein Indicators In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Adding extra hydrogen ions shifts. Find out its pk ind value, ph. Learn how phenolphthalein works as a weak acid indicator for titrations, and how its color changes with ph. See the structure,. Phenolphthalein Indicators.
From www.sciencephoto.com
Phenolphthalein Indicator Stock Image C030/7312 Science Photo Library Phenolphthalein Indicators Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid is colourless and its ion is bright pink. Learn about phenolphthalein, a ph indicator that turns pink in alkaline solutions and colorless in acidic and strongly alkaline solutions. Adding extra hydrogen ions shifts. Find out its pk ind value, ph. Learn. Phenolphthalein Indicators.
From www.sciencecompany.com
Phenolphthalein pH Indicator, 1 oz. Phenolphthalein Indicators See the structure, equilibrium, and ph range of phenolphthalein and other indicators. In this case, the weak acid is colourless and its ion is bright pink. Adding extra hydrogen ions shifts. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. As an indicator of a solution’s ph, phenolphthalein is colourless below ph 8.5 and attains a. Phenolphthalein Indicators.
From fineartamerica.com
Phenolphthalein Indicator Molecule Photograph by Molekuul/science Photo Library Fine Art America Phenolphthalein Indicators See the structure, equilibrium, and ph range of phenolphthalein and other indicators. Adding extra hydrogen ions shifts. Learn about phenolphthalein, a ph indicator that turns pink in alkaline solutions and colorless in acidic and strongly alkaline solutions. In this case, the weak acid is colourless and its ion is bright pink. Find out its pk ind value, ph. Phenolphthalein is. Phenolphthalein Indicators.
From www.britannica.com
Phenolphthalein pH indicator, acidbase titration, indicator dye Britannica Phenolphthalein Indicators As an indicator of a solution’s ph, phenolphthalein is colourless below ph 8.5 and attains a pink to deep red hue above ph 9.0. Adding extra hydrogen ions shifts. Find out its pk ind value, ph. Find out how to select, use and calculate the acid dissociation constants of indicators. Phenolphthalein is another commonly used indicator for titrations, and is. Phenolphthalein Indicators.
From
Phenolphthalein Indicators In this case, the weak acid is colourless and its ion is bright pink. See the structure, equilibrium, and ph range of phenolphthalein and other indicators. As an indicator of a solution’s ph, phenolphthalein is colourless below ph 8.5 and attains a pink to deep red hue above ph 9.0. Find out how to select, use and calculate the acid. Phenolphthalein Indicators.
From www.amazon.com
pH Indicator Set with Phenolphthalein, Universal Indicator, Bromothymol Blue Phenolphthalein Indicators Find out its pk ind value, ph. Find out how to select, use and calculate the acid dissociation constants of indicators. See the structure, equilibrium, and ph range of phenolphthalein and other indicators. Learn how phenolphthalein works as a weak acid indicator for titrations, and how its color changes with ph. Learn about phenolphthalein, a ph indicator that turns pink. Phenolphthalein Indicators.
From exysasktf.blob.core.windows.net
Phenolphthalein Indicator Color Base at Daryl White blog Phenolphthalein Indicators Learn about phenolphthalein, a ph indicator that turns pink in alkaline solutions and colorless in acidic and strongly alkaline solutions. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Find out its pk ind value, ph. Adding extra hydrogen ions shifts. Learn how phenolphthalein works as a weak acid indicator for titrations, and how its color. Phenolphthalein Indicators.
From
Phenolphthalein Indicators Find out how to select, use and calculate the acid dissociation constants of indicators. Learn how phenolphthalein works as a weak acid indicator for titrations, and how its color changes with ph. Find out its pk ind value, ph. In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly used indicator for. Phenolphthalein Indicators.
From
Phenolphthalein Indicators See the structure, equilibrium, and ph range of phenolphthalein and other indicators. In this case, the weak acid is colourless and its ion is bright pink. Find out its pk ind value, ph. Learn about phenolphthalein, a ph indicator that turns pink in alkaline solutions and colorless in acidic and strongly alkaline solutions. Adding extra hydrogen ions shifts. Phenolphthalein is. Phenolphthalein Indicators.
From www.youtube.com
Phenolphthalein Indicator Viva Questions pH range and Chemical Structure YouTube Phenolphthalein Indicators Find out how to select, use and calculate the acid dissociation constants of indicators. Learn about phenolphthalein, a ph indicator that turns pink in alkaline solutions and colorless in acidic and strongly alkaline solutions. As an indicator of a solution’s ph, phenolphthalein is colourless below ph 8.5 and attains a pink to deep red hue above ph 9.0. In this. Phenolphthalein Indicators.
From klaufzbrl.blob.core.windows.net
Phenolphthalein Indicator Range at Beatrice Simmons blog Phenolphthalein Indicators In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. See the structure, equilibrium, and ph range of phenolphthalein and other indicators. Adding extra hydrogen ions shifts. Learn about phenolphthalein, a ph indicator that turns pink in alkaline solutions and colorless in acidic. Phenolphthalein Indicators.
From labinsider.vn
Phenolphthalein ( Indicator grade, pure) Labinsider.vn Phenolphthalein Indicators Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid is colourless and its ion is bright pink. Find out how to select, use and calculate the acid dissociation constants of indicators. Learn about phenolphthalein, a ph indicator that turns pink in alkaline solutions and colorless in acidic and strongly alkaline. Phenolphthalein Indicators.
From
Phenolphthalein Indicators Find out how to select, use and calculate the acid dissociation constants of indicators. Learn how phenolphthalein works as a weak acid indicator for titrations, and how its color changes with ph. As an indicator of a solution’s ph, phenolphthalein is colourless below ph 8.5 and attains a pink to deep red hue above ph 9.0. Find out its pk. Phenolphthalein Indicators.
From www.watsons.ca
Phenolphthalein Indicator Watson's Barrels & Wine Making Supplies Phenolphthalein Indicators Adding extra hydrogen ions shifts. See the structure, equilibrium, and ph range of phenolphthalein and other indicators. As an indicator of a solution’s ph, phenolphthalein is colourless below ph 8.5 and attains a pink to deep red hue above ph 9.0. Find out its pk ind value, ph. Learn how phenolphthalein works as a weak acid indicator for titrations, and. Phenolphthalein Indicators.
From
Phenolphthalein Indicators As an indicator of a solution’s ph, phenolphthalein is colourless below ph 8.5 and attains a pink to deep red hue above ph 9.0. Learn how phenolphthalein works as a weak acid indicator for titrations, and how its color changes with ph. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Adding extra hydrogen ions shifts.. Phenolphthalein Indicators.
From
Phenolphthalein Indicators Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Adding extra hydrogen ions shifts. Find out its pk ind value, ph. Learn about phenolphthalein, a ph indicator that turns pink in alkaline solutions and colorless in acidic and strongly alkaline solutions. Learn how phenolphthalein works as a weak acid indicator for titrations, and how its color. Phenolphthalein Indicators.
From
Phenolphthalein Indicators Adding extra hydrogen ions shifts. Find out its pk ind value, ph. Find out how to select, use and calculate the acid dissociation constants of indicators. In this case, the weak acid is colourless and its ion is bright pink. Learn how phenolphthalein works as a weak acid indicator for titrations, and how its color changes with ph. See the. Phenolphthalein Indicators.
From www.alamy.com
Phenolphthalein indicator hires stock photography and images Alamy Phenolphthalein Indicators Learn about phenolphthalein, a ph indicator that turns pink in alkaline solutions and colorless in acidic and strongly alkaline solutions. See the structure, equilibrium, and ph range of phenolphthalein and other indicators. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. As an indicator of a solution’s ph, phenolphthalein is colourless below ph 8.5 and attains. Phenolphthalein Indicators.
From
Phenolphthalein Indicators Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid is colourless and its ion is bright pink. Adding extra hydrogen ions shifts. See the structure, equilibrium, and ph range of phenolphthalein and other indicators. Find out how to select, use and calculate the acid dissociation constants of indicators. Find out. Phenolphthalein Indicators.
From labbyag.es
Phenolphthalein Color Chart Labb by AG Phenolphthalein Indicators Find out its pk ind value, ph. Learn about phenolphthalein, a ph indicator that turns pink in alkaline solutions and colorless in acidic and strongly alkaline solutions. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Learn how phenolphthalein works as a weak acid indicator for titrations, and how its color changes with ph. As an. Phenolphthalein Indicators.
From
Phenolphthalein Indicators Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Find out its pk ind value, ph. See the structure, equilibrium, and ph range of phenolphthalein and other indicators. Adding extra hydrogen ions shifts. As an indicator of a solution’s ph, phenolphthalein is colourless below ph 8.5 and attains a pink to deep red hue above ph. Phenolphthalein Indicators.
From
Phenolphthalein Indicators Find out how to select, use and calculate the acid dissociation constants of indicators. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Find out its pk ind value, ph. In this case, the weak acid is colourless and its ion is bright pink. Learn about phenolphthalein, a ph indicator that turns pink in alkaline solutions. Phenolphthalein Indicators.