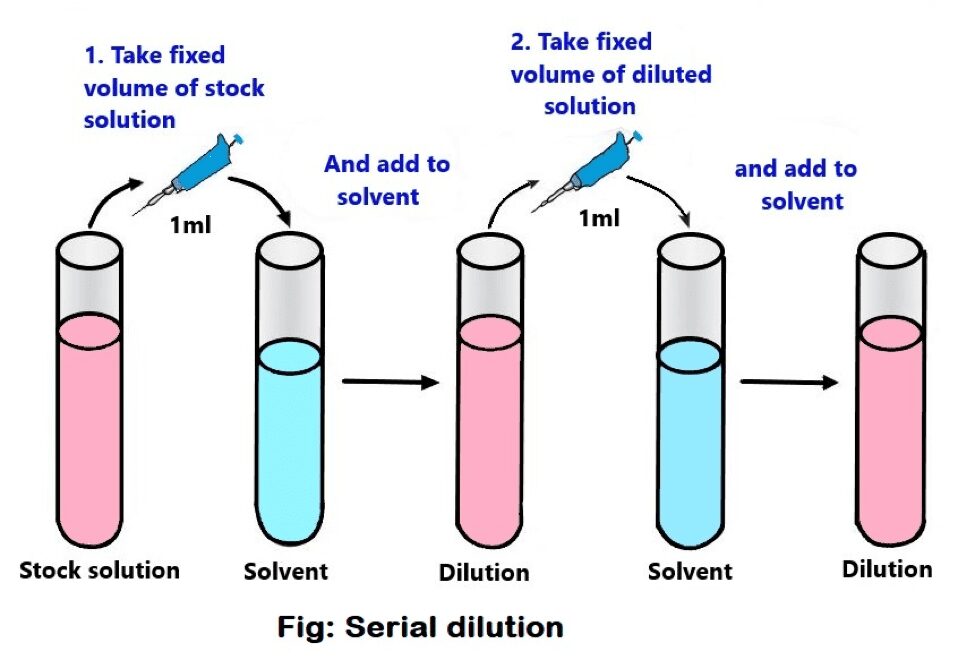
Dilutions Math . Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. Also calculate molarity, molality, mass fraction,. Compute the initial or final concentration or volume. Calculating dilution factors has never been easier! You can calculate the concentration of a solution following a dilution by applying this equation: A more simplified way of solving this is by using the dilution formula: There are many ways of expressing concentration and dilution. Explanations and examples of common methods. (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. Use the dilution equation or ideal dilution equation. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values.
from sciencequery.com
Calculating dilution factors has never been easier! There are many ways of expressing concentration and dilution. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A more simplified way of solving this is by using the dilution formula: M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. You can calculate the concentration of a solution following a dilution by applying this equation: Explanations and examples of common methods. (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. Compute the initial or final concentration or volume.
What is serial dilution method? And how to calculate? Science Query
Dilutions Math You can calculate the concentration of a solution following a dilution by applying this equation: You can calculate the concentration of a solution following a dilution by applying this equation: M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. Use the dilution equation or ideal dilution equation. Explanations and examples of common methods. Compute the initial or final concentration or volume. (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. Concentration is the removal of solvent, which increases the concentration of. Calculating dilution factors has never been easier! Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A more simplified way of solving this is by using the dilution formula: Also calculate molarity, molality, mass fraction,. There are many ways of expressing concentration and dilution.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilutions Math Compute the initial or final concentration or volume. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. Calculating dilution factors has never been easier! You can calculate. Dilutions Math.
From www.youtube.com
Microbiology Serial Dilution calculation YouTube Dilutions Math (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. You can calculate the concentration of a solution following a dilution by applying this equation: A more simplified. Dilutions Math.
From www.slideserve.com
PPT Solutions, concentrations dilutions PowerPoint Presentation, free Dilutions Math Use the dilution equation or ideal dilution equation. There are many ways of expressing concentration and dilution. Also calculate molarity, molality, mass fraction,. Compute the initial or final concentration or volume. You can calculate the concentration of a solution following a dilution by applying this equation: Calculating dilution factors has never been easier! A more simplified way of solving this. Dilutions Math.
From slidetodoc.com
Chapter 10 Dilution and Concentration Pharmaceutical Calculations for Dilutions Math Concentration is the removal of solvent, which increases the concentration of. Explanations and examples of common methods. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A more simplified way of solving this is by using the dilution formula: Calculating dilution factors has never been easier! You can calculate the concentration of a. Dilutions Math.
From yellowdig263.weebly.com
Serial Dilution Vs Parallel Dilution Dilutions Math You can calculate the concentration of a solution following a dilution by applying this equation: Explanations and examples of common methods. Use the dilution equation or ideal dilution equation. (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. Calculating dilution factors has never been easier! There are many ways of expressing concentration and dilution. M i. Dilutions Math.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilutions Math There are many ways of expressing concentration and dilution. Concentration is the removal of solvent, which increases the concentration of. (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. A more simplified way of solving this is by using the dilution formula: Also calculate molarity, molality, mass fraction,. Use the dilution equation or ideal dilution equation.. Dilutions Math.
From www.youtube.com
How solve dilution and concentration calculation problems YouTube Dilutions Math Compute the initial or final concentration or volume. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. You can calculate the concentration of a solution following a dilution by applying this equation: There are many ways of expressing concentration and dilution. Also calculate molarity, molality, mass fraction,. Use the dilution equation or ideal. Dilutions Math.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilutions Math Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. There are many ways of expressing concentration and dilution. Also calculate molarity, molality, mass fraction,. Calculating dilution factors has never been easier! (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. Concentration is the removal of solvent, which increases the. Dilutions Math.
From www.showme.com
Serial dilutions Math ShowMe Dilutions Math You can calculate the concentration of a solution following a dilution by applying this equation: Calculating dilution factors has never been easier! (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. A more simplified way of solving this is by using the dilution formula: Dilution is the addition of solvent, which decreases the concentration of the. Dilutions Math.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in Dilutions Math Compute the initial or final concentration or volume. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. Explanations and examples of common methods. Calculating. Dilutions Math.
From www.youtube.com
Dilution, solution, ratio, proportion (laboratory. calculations) YouTube Dilutions Math (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. There are many ways of expressing concentration and dilution. Compute the initial or final concentration or volume. You can calculate the concentration of a solution following a dilution by applying this equation: A more simplified way of solving this is by using the dilution formula: Use the. Dilutions Math.
From www.youtube.com
A Level Chemistry Dilution Calculations Worked Example YouTube Dilutions Math Use the dilution equation or ideal dilution equation. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. Explanations and examples of common methods. There are many ways of expressing concentration and dilution. Also calculate molarity, molality, mass fraction,. Dilution is. Dilutions Math.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved Dilutions Math (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. Calculating dilution factors has never been easier! Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Compute the initial or final concentration or volume. There are many ways of expressing concentration and dilution. You can calculate the concentration of a. Dilutions Math.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilutions Math (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. Explanations and examples of common methods. You can calculate the concentration of a solution following a dilution by applying this equation: Calculating dilution factors has never been easier! Also calculate molarity, molality, mass fraction,. Use the dilution equation or ideal dilution equation. Concentration is the removal of. Dilutions Math.
From www.showme.com
Calculating Serial Dilution Math ShowMe Dilutions Math A more simplified way of solving this is by using the dilution formula: There are many ways of expressing concentration and dilution. Calculating dilution factors has never been easier! Compute the initial or final concentration or volume. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. Dilutions Math.
From www.slideserve.com
PPT 03 Concentration DILUTIONS PowerPoint Presentation, free Dilutions Math Also calculate molarity, molality, mass fraction,. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Explanations and examples of common methods. (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. M i v i = m f v f where m is molarity, v is volume, and the subscripts. Dilutions Math.
From www.youtube.com
Calculating Dilutions C1V1=C2V2 PowerPoint YouTube Dilutions Math Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. Also calculate molarity, molality, mass fraction,. Concentration is the removal of solvent, which increases the concentration of. A more simplified way of solving this is by using the dilution formula: M. Dilutions Math.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Dilutions Math Concentration is the removal of solvent, which increases the concentration of. Explanations and examples of common methods. Compute the initial or final concentration or volume. Also calculate molarity, molality, mass fraction,. A more simplified way of solving this is by using the dilution formula: Use the dilution equation or ideal dilution equation. (m1) (v1) = (m2) (v2), where m's are. Dilutions Math.
From www.youtube.com
Serial Dilution Methods & Calaculations YouTube Dilutions Math M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Use the dilution equation or ideal dilution equation. Explanations and examples of common methods. (m1). Dilutions Math.
From www.youtube.com
Dilutions in clinical chemistry the math in detail YouTube Dilutions Math (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. You can calculate the concentration of a solution following a dilution by applying this equation: Explanations and examples of common methods. Use the dilution equation or ideal dilution equation. A more simplified way of solving this is by using the dilution formula: M i v i =. Dilutions Math.
From afzanituall.blogspot.com
Dilutions Worksheet Dilutions Worksheet Free division worksheets Dilutions Math A more simplified way of solving this is by using the dilution formula: Concentration is the removal of solvent, which increases the concentration of. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. You can calculate the concentration of a. Dilutions Math.
From www.youtube.com
DILUTIONS PART 3 calculating amounts C1V1=C2V2 YouTube Dilutions Math Also calculate molarity, molality, mass fraction,. You can calculate the concentration of a solution following a dilution by applying this equation: Compute the initial or final concentration or volume. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. Explanations and. Dilutions Math.
From www.researchgate.net
Preparation of Dilutions Download Table Dilutions Math Concentration is the removal of solvent, which increases the concentration of. Compute the initial or final concentration or volume. Calculating dilution factors has never been easier! Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. You can calculate the concentration. Dilutions Math.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilutions Math M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. Compute the initial or final concentration or volume. Explanations and examples of common methods. Calculating dilution factors has. Dilutions Math.
From www.slideserve.com
PPT Math for the Pharmacy Technician Concepts and Calculations Dilutions Math There are many ways of expressing concentration and dilution. You can calculate the concentration of a solution following a dilution by applying this equation: A more simplified way of solving this is by using the dilution formula: Also calculate molarity, molality, mass fraction,. M i v i = m f v f where m is molarity, v is volume, and. Dilutions Math.
From www.showme.com
Dependent Dilutions Math ShowMe Dilutions Math You can calculate the concentration of a solution following a dilution by applying this equation: Explanations and examples of common methods. Compute the initial or final concentration or volume. (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. Also calculate molarity, molality, mass fraction,. Concentration is the removal of solvent, which increases the concentration of. There. Dilutions Math.
From www.slideserve.com
PPT Lesson 18 PowerPoint Presentation, free download ID3739399 Dilutions Math Also calculate molarity, molality, mass fraction,. Compute the initial or final concentration or volume. There are many ways of expressing concentration and dilution. Calculating dilution factors has never been easier! Explanations and examples of common methods. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. You can calculate the concentration of a solution. Dilutions Math.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilutions Math Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Calculating dilution factors has never been easier! M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. Use the dilution equation or ideal dilution equation.. Dilutions Math.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube Dilutions Math Concentration is the removal of solvent, which increases the concentration of. Compute the initial or final concentration or volume. Calculating dilution factors has never been easier! Use the dilution equation or ideal dilution equation. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial. Dilutions Math.
From www.youtube.com
GeeklyHub Dilution of Solution Dilution Calculations & Problems Dilutions Math Calculating dilution factors has never been easier! Use the dilution equation or ideal dilution equation. Compute the initial or final concentration or volume. (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to. Dilutions Math.
From www.youtube.com
Dilution Calculation Practice YouTube Dilutions Math Also calculate molarity, molality, mass fraction,. There are many ways of expressing concentration and dilution. Compute the initial or final concentration or volume. (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. Use the dilution equation or ideal dilution equation. You can calculate the concentration of a solution following a dilution by applying this equation: Dilution. Dilutions Math.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilutions Math There are many ways of expressing concentration and dilution. Explanations and examples of common methods. Compute the initial or final concentration or volume. Also calculate molarity, molality, mass fraction,. (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. You can calculate the concentration of a solution following a dilution by applying this equation: A more simplified. Dilutions Math.
From www.chegg.com
Solved In The Image Above, The Final Dilution Is 1 You M... Dilutions Math Explanations and examples of common methods. Calculating dilution factors has never been easier! A more simplified way of solving this is by using the dilution formula: (m1) (v1) = (m2) (v2), where m's are molarities and v's are volumes. Compute the initial or final concentration or volume. You can calculate the concentration of a solution following a dilution by applying. Dilutions Math.
From klafwpnrs.blob.core.windows.net
Dilution Formula Explanation at John Schell blog Dilutions Math Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. You can calculate the concentration of a solution following a dilution by applying this equation: Also calculate molarity, molality, mass fraction,. There are many ways of expressing concentration and dilution. Use the dilution equation or ideal dilution equation. Calculating dilution factors has never been. Dilutions Math.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples Dilutions Math There are many ways of expressing concentration and dilution. A more simplified way of solving this is by using the dilution formula: Also calculate molarity, molality, mass fraction,. Concentration is the removal of solvent, which increases the concentration of. Use the dilution equation or ideal dilution equation. Explanations and examples of common methods. You can calculate the concentration of a. Dilutions Math.