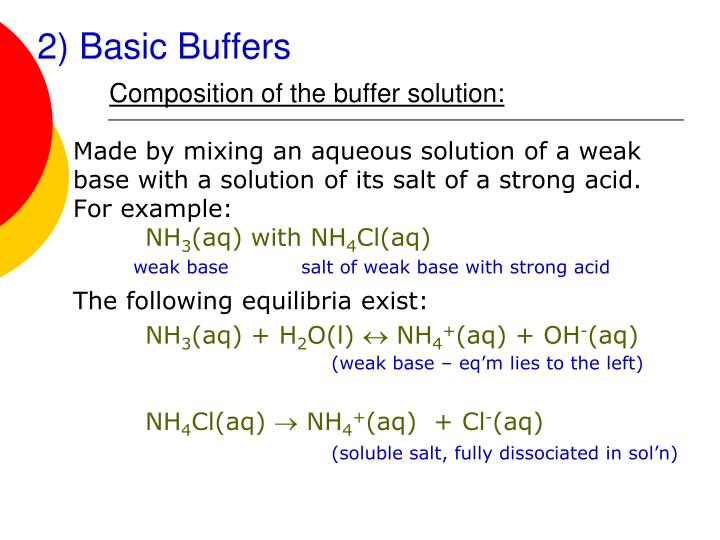
Base Added To Buffer Solution . A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. The acid and its conjugate base. A solution of acetic acid. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). Buffers are solutions that resist a change in ph after adding an acid or a base. If you add a base to a buffer solution, the hydrogen ion concentration decreases by less than the amount expected for the quantity of base added. It is able to neutralize small. Buffers contain a weak acid (\(ha\)) and its.
from www.slideserve.com
The acid and its conjugate base. If you add a base to a buffer solution, the hydrogen ion concentration decreases by less than the amount expected for the quantity of base added. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A solution of acetic acid. Buffers are solutions that resist a change in ph after adding an acid or a base. Buffers contain a weak acid (\(ha\)) and its.
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint
Base Added To Buffer Solution Buffers are solutions that resist a change in ph after adding an acid or a base. Buffers are solutions that resist a change in ph after adding an acid or a base. A solution of acetic acid. The acid and its conjugate base. If you add a base to a buffer solution, the hydrogen ion concentration decreases by less than the amount expected for the quantity of base added. Buffers contain a weak acid (\(ha\)) and its. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). It is able to neutralize small. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk Base Added To Buffer Solution The acid and its conjugate base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A solution of acetic acid. If you add a base to a buffer solution, the hydrogen ion concentration decreases by less than the amount expected for the quantity of base added. It is able to. Base Added To Buffer Solution.
From jackwestin.com
Buffers 2 Acid Base Equilibria MCAT Content Base Added To Buffer Solution A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. If you add a base to a buffer solution, the hydrogen ion concentration decreases by less than the amount expected for the quantity of base added. Buffer solutions resist a change in ph. Base Added To Buffer Solution.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation ID496487 Base Added To Buffer Solution A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. Buffer solutions resist a change in ph when. Base Added To Buffer Solution.
From www.brainkart.com
Buffers Base Added To Buffer Solution Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). It is able to neutralize small. If you add a base to a buffer solution, the hydrogen ion concentration decreases by less than the amount expected for the quantity of base added. Buffers are solutions that resist a. Base Added To Buffer Solution.
From allyson-has-mccoy.blogspot.com
Which Pair Will Produce a Buffer Solution AllysonhasMccoy Base Added To Buffer Solution A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The acid and its conjugate base. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). If you add a base to a buffer solution, the hydrogen ion concentration. Base Added To Buffer Solution.
From musicbykatie.com
Does Nh4Oh And Hcl Form Buffer? 28 Most Correct Answers Base Added To Buffer Solution If you add a base to a buffer solution, the hydrogen ion concentration decreases by less than the amount expected for the quantity of base added. Buffers are solutions that resist a change in ph after adding an acid or a base. A solution of acetic acid. Buffers contain a weak acid (\(ha\)) and its. A mixture of a weak. Base Added To Buffer Solution.
From www.slideshare.net
2012 topic 18 2 buffer solutions Base Added To Buffer Solution A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The acid and its conjugate base. A solution of acetic acid. Buffers are solutions that resist a change in ph after adding an acid or a base. A mixture of a weak acid and its conjugate base (or a mixture of. Base Added To Buffer Solution.
From general.chemistrysteps.com
Buffer Solutions Chemistry Steps Base Added To Buffer Solution A solution of acetic acid. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers contain a weak acid (\(ha\)) and its. Buffers. Base Added To Buffer Solution.
From design.udlvirtual.edu.pe
What Is The Ph Of A Buffer Solution Design Talk Base Added To Buffer Solution It is able to neutralize small. The acid and its conjugate base. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Buffers contain a weak acid (\(ha\)) and its. Buffer solutions resist a change in ph when small amounts of a strong. Base Added To Buffer Solution.
From www.slideserve.com
PPT Chapter 17 Additional Aspects of Aqueous Equilibria PowerPoint Base Added To Buffer Solution Buffers contain a weak acid (\(ha\)) and its. The acid and its conjugate base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. Buffers are solutions that resist a change in ph after adding an acid or a base. Buffer solutions resist a change. Base Added To Buffer Solution.
From www.youtube.com
Adding strong base to a buffer solution YouTube Base Added To Buffer Solution Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). It is able to neutralize small. A solution of acetic acid. If you add a base to a buffer solution, the hydrogen ion concentration decreases by less than the amount expected for the quantity of base added. Buffers. Base Added To Buffer Solution.
From www.chegg.com
1 point ∞ In this experiment, we started with two Base Added To Buffer Solution A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers are solutions that resist a change in ph after adding an acid or. Base Added To Buffer Solution.
From slideplayer.com
SCH4U Acids and Bases BUFFER SOLUTIONS. ppt download Base Added To Buffer Solution It is able to neutralize small. Buffers are solutions that resist a change in ph after adding an acid or a base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. If you add a base to a buffer solution, the hydrogen ion concentration decreases by less than the amount. Base Added To Buffer Solution.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Base Added To Buffer Solution A solution of acetic acid. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. If you add a base to a buffer solution, the hydrogen ion concentration decreases by less than the amount expected for the quantity of base added. A buffer. Base Added To Buffer Solution.
From www.chegg.com
Solved When an acid or a base is added to a buffer, Base Added To Buffer Solution Buffers are solutions that resist a change in ph after adding an acid or a base. If you add a base to a buffer solution, the hydrogen ion concentration decreases by less than the amount expected for the quantity of base added. Buffers contain a weak acid (\(ha\)) and its. A mixture of a weak acid and its conjugate base. Base Added To Buffer Solution.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs Base Added To Buffer Solution Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). The acid and its conjugate base. Buffers are solutions that resist a change in ph after adding an acid or a base. A buffer is a solution that can resist ph change upon the addition of an acidic. Base Added To Buffer Solution.
From general.chemistrysteps.com
pH of a Buffer Solution Chemistry Steps Base Added To Buffer Solution Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). Buffers are solutions that resist a change in ph after adding an acid or a base. The acid and its conjugate base. A solution of acetic acid. A buffer is a solution that can resist ph change upon. Base Added To Buffer Solution.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Base Added To Buffer Solution The acid and its conjugate base. Buffers are solutions that resist a change in ph after adding an acid or a base. It is able to neutralize small. If you add a base to a buffer solution, the hydrogen ion concentration decreases by less than the amount expected for the quantity of base added. A buffer is a solution that. Base Added To Buffer Solution.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Base Added To Buffer Solution Buffers contain a weak acid (\(ha\)) and its. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). The acid and its conjugate base. If you add a base to a buffer solution, the hydrogen ion concentration decreases by less than the amount expected for the quantity of. Base Added To Buffer Solution.
From www.brainkart.com
How do buffers work? Base Added To Buffer Solution A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Buffers contain a weak acid (\(ha\)) and its. Buffer solutions resist a change in. Base Added To Buffer Solution.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Base Added To Buffer Solution A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Buffers are solutions that resist a change in ph after adding an acid or. Base Added To Buffer Solution.
From www.coursehero.com
[Solved] 1. Explain what is in a buffer. Discuss the function of a Base Added To Buffer Solution If you add a base to a buffer solution, the hydrogen ion concentration decreases by less than the amount expected for the quantity of base added. The acid and its conjugate base. A solution of acetic acid. Buffers contain a weak acid (\(ha\)) and its. A mixture of a weak acid and its conjugate base (or a mixture of a. Base Added To Buffer Solution.
From byjus.com
When a small amount of HCL is added to a buffer solution of acetic acid Base Added To Buffer Solution It is able to neutralize small. The acid and its conjugate base. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer solutions. Base Added To Buffer Solution.
From www.slideserve.com
PPT Weak acids and bases Salt of weak acid and bases buffer Lecture 9 Base Added To Buffer Solution It is able to neutralize small. Buffers contain a weak acid (\(ha\)) and its. If you add a base to a buffer solution, the hydrogen ion concentration decreases by less than the amount expected for the quantity of base added. The acid and its conjugate base. A mixture of a weak acid and its conjugate base (or a mixture of. Base Added To Buffer Solution.
From www.slideserve.com
PPT Chemistry 100 Chapter 17 PowerPoint Presentation, free download Base Added To Buffer Solution A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. If you add a base to a buffer solution, the hydrogen ion concentration decreases by less than the amount expected for the quantity of base added. Buffers contain a weak acid (\(ha\)) and. Base Added To Buffer Solution.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Base Added To Buffer Solution The acid and its conjugate base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers contain a weak acid (\(ha\)) and its. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or.. Base Added To Buffer Solution.
From scienceinfo.com
Buffer Solution Types, Properties, and Uses Base Added To Buffer Solution A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. It is able to neutralize small. A solution of acetic acid. The acid and its conjugate base. If you add a base to a buffer solution, the hydrogen ion concentration decreases by less. Base Added To Buffer Solution.
From www.slideserve.com
PPT DEFINITIONS OF ACIDS & BASES PowerPoint Presentation, free Base Added To Buffer Solution A solution of acetic acid. Buffers contain a weak acid (\(ha\)) and its. If you add a base to a buffer solution, the hydrogen ion concentration decreases by less than the amount expected for the quantity of base added. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture. Base Added To Buffer Solution.
From magmastory.blogspot.com
Which Of The Following Represents A Buffer System? magmastory Base Added To Buffer Solution Buffers are solutions that resist a change in ph after adding an acid or a base. A solution of acetic acid. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). It is able to neutralize small. The acid and its conjugate base. If you add a base. Base Added To Buffer Solution.
From chem.libretexts.org
8.8 Buffers Solutions That Resist pH Change Chemistry LibreTexts Base Added To Buffer Solution If you add a base to a buffer solution, the hydrogen ion concentration decreases by less than the amount expected for the quantity of base added. Buffers are solutions that resist a change in ph after adding an acid or a base. Buffers contain a weak acid (\(ha\)) and its. The acid and its conjugate base. A buffer is a. Base Added To Buffer Solution.
From skc13chem.blogspot.com
SKC year 13 Chemistry Buffer solutions Base Added To Buffer Solution A solution of acetic acid. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). Buffers contain a weak acid (\(ha\)) and its. If you add a base to a buffer solution, the hydrogen ion concentration decreases by less than the amount expected for the quantity of base. Base Added To Buffer Solution.
From studylib.net
Buffer Solutions Base Added To Buffer Solution The acid and its conjugate base. It is able to neutralize small. A solution of acetic acid. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution that can resist ph change upon the addition of an acidic. Base Added To Buffer Solution.
From mappingmemories.ca
Autorizar Vandalir Psicologicamente buffer ph calculator Pigmento Base Added To Buffer Solution Buffers contain a weak acid (\(ha\)) and its. It is able to neutralize small. The acid and its conjugate base. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Buffers are solutions that resist a change in ph after adding an acid. Base Added To Buffer Solution.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Base Added To Buffer Solution The acid and its conjugate base. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A solution of acetic acid. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. If you add. Base Added To Buffer Solution.
From courses.lumenlearning.com
Buffers Chemistry for Majors Base Added To Buffer Solution Buffers contain a weak acid (\(ha\)) and its. A solution of acetic acid. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). Buffers are solutions that resist a. Base Added To Buffer Solution.