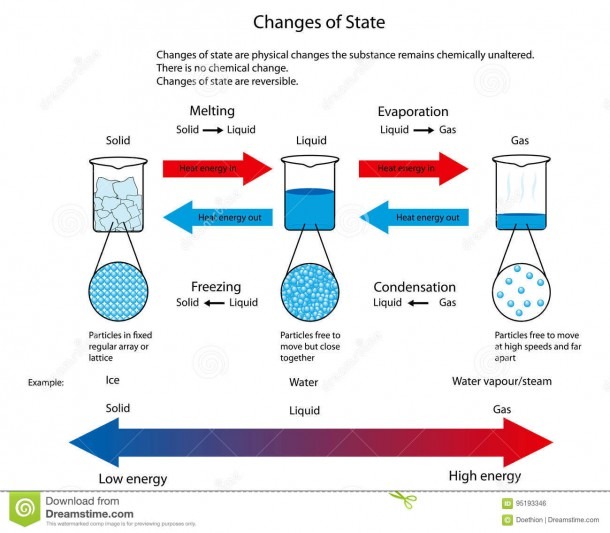
Solid To Liquid Conversion . The energy required to melt 1 mol of a substance is its enthalpy of fusion (δ h fus ). After learning about changing states of matter, and conversion from solid to liquid, let us now have a look at the melting point. to convert a solid into a liquid you need to heat the solid beyond its melting point. the process of obtaining solid from the liquid is the opposite; Atoms, ions, and molecules in a solid pack tightly together and may form. A solid is a state of matter with a defined shape and volume. The atoms absorb this heat and vibrate more. the process by which a substance changes from the liquid phase to the solid phase is known as freezing. Remove heat from the sample and it is called fusion or freezing. The energy change required to vaporize 1 mol of a substance is the enthalpy of vaporization (δ h vap ). The conversion of a solid to a liquid is called fusion (or melting). The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). the conversion of a solid to a liquid is called fusion (or melting).
from www.mikrora.com
After learning about changing states of matter, and conversion from solid to liquid, let us now have a look at the melting point. Remove heat from the sample and it is called fusion or freezing. The energy change required to vaporize 1 mol of a substance is the enthalpy of vaporization (δ h vap ). to convert a solid into a liquid you need to heat the solid beyond its melting point. The conversion of a solid to a liquid is called fusion (or melting). A solid is a state of matter with a defined shape and volume. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). the process of obtaining solid from the liquid is the opposite; The atoms absorb this heat and vibrate more. the process by which a substance changes from the liquid phase to the solid phase is known as freezing.
Illustration For Changes Of State Between Solid, Liquid And Gas Best
Solid To Liquid Conversion The conversion of a solid to a liquid is called fusion (or melting). A solid is a state of matter with a defined shape and volume. The energy change required to vaporize 1 mol of a substance is the enthalpy of vaporization (δ h vap ). Atoms, ions, and molecules in a solid pack tightly together and may form. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δ h fus ). to convert a solid into a liquid you need to heat the solid beyond its melting point. After learning about changing states of matter, and conversion from solid to liquid, let us now have a look at the melting point. the process by which a substance changes from the liquid phase to the solid phase is known as freezing. Remove heat from the sample and it is called fusion or freezing. the process of obtaining solid from the liquid is the opposite; The conversion of a solid to a liquid is called fusion (or melting). The atoms absorb this heat and vibrate more. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). the conversion of a solid to a liquid is called fusion (or melting).
From www.animalia-life.club
Liquid Measurement Conversion Chart Liter Solid To Liquid Conversion The atoms absorb this heat and vibrate more. to convert a solid into a liquid you need to heat the solid beyond its melting point. the process by which a substance changes from the liquid phase to the solid phase is known as freezing. After learning about changing states of matter, and conversion from solid to liquid, let. Solid To Liquid Conversion.
From www.youtube.com
Measurement conversions of liquids and solids YouTube Solid To Liquid Conversion The atoms absorb this heat and vibrate more. the process by which a substance changes from the liquid phase to the solid phase is known as freezing. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δ h fus ). Atoms, ions, and molecules in a solid pack tightly together and may form.. Solid To Liquid Conversion.
From samson-jolpblogsantos.blogspot.com
How Does Temperature Affect Solids Liquids and Gases Solid To Liquid Conversion The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The atoms absorb this heat and vibrate more. Atoms, ions, and molecules in a solid pack tightly together and may form. the conversion of a solid to a liquid is called fusion (or melting). The energy change required to vaporize 1 mol. Solid To Liquid Conversion.
From sciencenotes.org
States of Matter Solid To Liquid Conversion The conversion of a solid to a liquid is called fusion (or melting). The energy change required to vaporize 1 mol of a substance is the enthalpy of vaporization (δ h vap ). A solid is a state of matter with a defined shape and volume. Atoms, ions, and molecules in a solid pack tightly together and may form. The. Solid To Liquid Conversion.
From www.snexplores.org
Explainer What are the different states of matter? Solid To Liquid Conversion The energy required to melt 1 mol of a substance is its enthalpy of fusion (δ h fus ). Remove heat from the sample and it is called fusion or freezing. After learning about changing states of matter, and conversion from solid to liquid, let us now have a look at the melting point. A solid is a state of. Solid To Liquid Conversion.
From www.savemyexams.com
The Three States of Matter Oxford AQA IGCSE Chemistry Revision Notes 2016 Solid To Liquid Conversion Remove heat from the sample and it is called fusion or freezing. A solid is a state of matter with a defined shape and volume. After learning about changing states of matter, and conversion from solid to liquid, let us now have a look at the melting point. to convert a solid into a liquid you need to heat. Solid To Liquid Conversion.
From slgas03.blogspot.com
solid,liquid and gas States of Matter Solid To Liquid Conversion The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Remove heat from the sample and it is called fusion or freezing. The conversion of a solid to a liquid is called fusion (or melting). The atoms absorb this heat and vibrate more. The energy required to melt 1 mol of a substance. Solid To Liquid Conversion.
From dxoktdxhp.blob.core.windows.net
From Solid To Liquid Examples at Lauren Arthur blog Solid To Liquid Conversion The conversion of a solid to a liquid is called fusion (or melting). The atoms absorb this heat and vibrate more. A solid is a state of matter with a defined shape and volume. the process of obtaining solid from the liquid is the opposite; The energy change required to vaporize 1 mol of a substance is the enthalpy. Solid To Liquid Conversion.
From sciencenotes.org
10 Examples of Solids, Liquids, Gases, and Plasma Solid To Liquid Conversion the process by which a substance changes from the liquid phase to the solid phase is known as freezing. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δ h fus ). the conversion of a solid to a liquid is called fusion (or melting). The energy required to melt 1 mol. Solid To Liquid Conversion.
From circuitlibrarybauer.z13.web.core.windows.net
Solid Liquid Gas Diagram Solid To Liquid Conversion the process by which a substance changes from the liquid phase to the solid phase is known as freezing. The atoms absorb this heat and vibrate more. the conversion of a solid to a liquid is called fusion (or melting). to convert a solid into a liquid you need to heat the solid beyond its melting point.. Solid To Liquid Conversion.
From templatelab.com
45 Printable Liquid Measurements Charts [Liquid Conversion] ᐅ TemplateLab Solid To Liquid Conversion the conversion of a solid to a liquid is called fusion (or melting). The conversion of a solid to a liquid is called fusion (or melting). to convert a solid into a liquid you need to heat the solid beyond its melting point. The atoms absorb this heat and vibrate more. the process of obtaining solid from. Solid To Liquid Conversion.
From www.theschoolrun.com
What are states of matter? TheSchoolRun Solid To Liquid Conversion The conversion of a solid to a liquid is called fusion (or melting). The atoms absorb this heat and vibrate more. The energy change required to vaporize 1 mol of a substance is the enthalpy of vaporization (δ h vap ). the process by which a substance changes from the liquid phase to the solid phase is known as. Solid To Liquid Conversion.
From www.mikrora.com
Illustration For Changes Of State Between Solid, Liquid And Gas Best Solid To Liquid Conversion the process of obtaining solid from the liquid is the opposite; The energy change required to vaporize 1 mol of a substance is the enthalpy of vaporization (δ h vap ). to convert a solid into a liquid you need to heat the solid beyond its melting point. After learning about changing states of matter, and conversion from. Solid To Liquid Conversion.
From online-learning-college.com
States of matter solids, liquids and gases Interconversions Solid To Liquid Conversion Remove heat from the sample and it is called fusion or freezing. The atoms absorb this heat and vibrate more. The energy change required to vaporize 1 mol of a substance is the enthalpy of vaporization (δ h vap ). The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Atoms, ions, and. Solid To Liquid Conversion.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Solid To Liquid Conversion The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). After learning about changing states of matter, and conversion from solid to liquid, let us now have a look at the melting point. Atoms, ions, and molecules in a solid pack tightly together and may form. the process by which a substance. Solid To Liquid Conversion.
From www.researchgate.net
Schematic diagram of the gasliquidsolid conversion (a); the Solid To Liquid Conversion The conversion of a solid to a liquid is called fusion (or melting). the process by which a substance changes from the liquid phase to the solid phase is known as freezing. A solid is a state of matter with a defined shape and volume. the process of obtaining solid from the liquid is the opposite; the. Solid To Liquid Conversion.
From www.pinterest.com
Be A Better Chef With These Simple Cooking Tips *** Learn more by Solid To Liquid Conversion Atoms, ions, and molecules in a solid pack tightly together and may form. the process by which a substance changes from the liquid phase to the solid phase is known as freezing. The conversion of a solid to a liquid is called fusion (or melting). the process of obtaining solid from the liquid is the opposite; The energy. Solid To Liquid Conversion.
From za.pinterest.com
Solids, liquids and gases PrimaryLeap.co.uk Science Chart, Science Solid To Liquid Conversion the conversion of a solid to a liquid is called fusion (or melting). The energy required to melt 1 mol of a substance is its enthalpy of fusion (δ h fus ). to convert a solid into a liquid you need to heat the solid beyond its melting point. the process of obtaining solid from the liquid. Solid To Liquid Conversion.
From www.researchgate.net
Mechanism schematic diagram of (A) solidliquid conversion, (B Solid To Liquid Conversion The atoms absorb this heat and vibrate more. A solid is a state of matter with a defined shape and volume. Remove heat from the sample and it is called fusion or freezing. After learning about changing states of matter, and conversion from solid to liquid, let us now have a look at the melting point. to convert a. Solid To Liquid Conversion.
From www.math-salamanders.com
Liquid Conversion Chart UK Measures Solid To Liquid Conversion The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Remove heat from the sample and it is called fusion or freezing. The energy change required to vaporize 1 mol of a substance is the enthalpy of vaporization (δ h vap ). the process of obtaining solid from the liquid is the. Solid To Liquid Conversion.
From lessonschoolemirates.z13.web.core.windows.net
Printable Liquid Measurement Conversion Chart Solid To Liquid Conversion After learning about changing states of matter, and conversion from solid to liquid, let us now have a look at the melting point. the conversion of a solid to a liquid is called fusion (or melting). The conversion of a solid to a liquid is called fusion (or melting). The atoms absorb this heat and vibrate more. A solid. Solid To Liquid Conversion.
From www.teachoo.com
Interconversion of States of Matter with Flow Chart Teachoo Solid To Liquid Conversion Remove heat from the sample and it is called fusion or freezing. the process by which a substance changes from the liquid phase to the solid phase is known as freezing. After learning about changing states of matter, and conversion from solid to liquid, let us now have a look at the melting point. The energy required to melt. Solid To Liquid Conversion.
From saylordotorg.github.io
Solids and Liquids Solid To Liquid Conversion the conversion of a solid to a liquid is called fusion (or melting). The energy required to melt 1 mol of a substance is its enthalpy of fusion (δ h fus ). A solid is a state of matter with a defined shape and volume. The atoms absorb this heat and vibrate more. The energy required to melt 1. Solid To Liquid Conversion.
From www.gasrebate.net
Properties Of Solids Liquids Gases Compared Teachoo Science Solid To Liquid Conversion A solid is a state of matter with a defined shape and volume. the conversion of a solid to a liquid is called fusion (or melting). Atoms, ions, and molecules in a solid pack tightly together and may form. The conversion of a solid to a liquid is called fusion (or melting). the process of obtaining solid from. Solid To Liquid Conversion.
From dxoarnayy.blob.core.windows.net
Pictures Of Particles In Solids Liquids And Gases at Barbara Wilson blog Solid To Liquid Conversion The atoms absorb this heat and vibrate more. Remove heat from the sample and it is called fusion or freezing. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). to convert a solid into a liquid you need to heat the solid beyond its melting point. The energy change required to. Solid To Liquid Conversion.
From www.pinterest.com
Liquid and solid conversions Kitchen Pinterest Solid To Liquid Conversion A solid is a state of matter with a defined shape and volume. The energy change required to vaporize 1 mol of a substance is the enthalpy of vaporization (δ h vap ). After learning about changing states of matter, and conversion from solid to liquid, let us now have a look at the melting point. The atoms absorb this. Solid To Liquid Conversion.
From www.researchgate.net
Inducing the SolidLiquid Conversion of Zinc Metal Anode in Alkaline Solid To Liquid Conversion The atoms absorb this heat and vibrate more. The energy change required to vaporize 1 mol of a substance is the enthalpy of vaporization (δ h vap ). The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). A solid is a state of matter with a defined shape and volume. Atoms, ions,. Solid To Liquid Conversion.
From www.slideshare.net
Solids, liquids and_gases PPT Solid To Liquid Conversion A solid is a state of matter with a defined shape and volume. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δ h fus ). The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The conversion of a solid to a liquid is called fusion. Solid To Liquid Conversion.
From energy-models.com
Gases & Liquids (Fluids) and Solids Solid To Liquid Conversion the process by which a substance changes from the liquid phase to the solid phase is known as freezing. the conversion of a solid to a liquid is called fusion (or melting). The energy change required to vaporize 1 mol of a substance is the enthalpy of vaporization (δ h vap ). A solid is a state of. Solid To Liquid Conversion.
From mungfali.com
Liquid Conversion Chart Printable Solid To Liquid Conversion The atoms absorb this heat and vibrate more. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). the conversion of a solid to a liquid is called fusion (or melting). to convert a solid into a liquid you need to heat the solid beyond its melting point. the process. Solid To Liquid Conversion.
From www.alamy.com
Water States of matter Phase. Change of State for Water Diagram Solid To Liquid Conversion The atoms absorb this heat and vibrate more. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). A solid is a state of matter with a defined shape and volume. the process by which a substance changes from the liquid phase to the solid phase is known as freezing. The conversion. Solid To Liquid Conversion.
From davida.davivienda.com
Printable Liquid Conversion Chart Printable Word Searches Solid To Liquid Conversion the process by which a substance changes from the liquid phase to the solid phase is known as freezing. A solid is a state of matter with a defined shape and volume. the process of obtaining solid from the liquid is the opposite; The energy required to melt 1 mol of a substance is its enthalpy of fusion. Solid To Liquid Conversion.
From studyunmeriting.z21.web.core.windows.net
7 Cups Is How Many Liters Solid To Liquid Conversion Remove heat from the sample and it is called fusion or freezing. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δ h fus ). The energy change required to vaporize 1 mol of a substance is the enthalpy of vaporization (δ h vap ). A solid is a state of matter with a. Solid To Liquid Conversion.
From www.thespruceeats.com
Liquid Measurement Conversion Chart for Cooking Solid To Liquid Conversion The energy required to melt 1 mol of a substance is its enthalpy of fusion (δ h fus ). the process by which a substance changes from the liquid phase to the solid phase is known as freezing. After learning about changing states of matter, and conversion from solid to liquid, let us now have a look at the. Solid To Liquid Conversion.
From www.slideshare.net
Solids, liquids and_gases PPT Solid To Liquid Conversion Remove heat from the sample and it is called fusion or freezing. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). A solid is a state of matter with a defined shape and volume. to convert a solid into a liquid you need to heat the solid beyond its melting point.. Solid To Liquid Conversion.