Evaporation Intermolecular Forces . Explain how intermolecular forces affect rates of vaporization, evaporation, and condensation. (attractions between the molecules) in the liquid state have to be broken to. A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. Using the language of intermolecular forces, explain the order of the evaporation rates you observed in the first part of your experiment. This evaporation is an endothermic process that results in a temperature. Evaporation occurs when the probe is removed from the liquidõs container. Why is evaporation an endothermic process? In order of decreasing strength, the important. The larger the intermolecular forces in a compound, the slower its evaporation rate. The stronger these forces, the lower. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present.
from slidetodoc.com
A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. The larger the intermolecular forces in a compound, the slower its evaporation rate. This evaporation is an endothermic process that results in a temperature. Evaporation occurs when the probe is removed from the liquidõs container. The stronger these forces, the lower. (attractions between the molecules) in the liquid state have to be broken to. In order of decreasing strength, the important. Why is evaporation an endothermic process? To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Explain how intermolecular forces affect rates of vaporization, evaporation, and condensation.
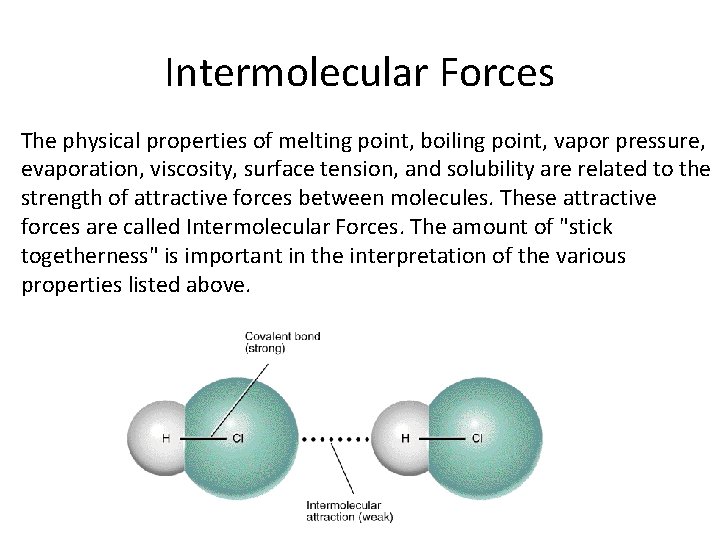
Intermolecular Forces The physical properties of melting point
Evaporation Intermolecular Forces To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. (attractions between the molecules) in the liquid state have to be broken to. Using the language of intermolecular forces, explain the order of the evaporation rates you observed in the first part of your experiment. The larger the intermolecular forces in a compound, the slower its evaporation rate. Explain how intermolecular forces affect rates of vaporization, evaporation, and condensation. In order of decreasing strength, the important. Why is evaporation an endothermic process? To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Evaporation occurs when the probe is removed from the liquidõs container. A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. The stronger these forces, the lower. This evaporation is an endothermic process that results in a temperature.
From www.slideserve.com
PPT 14.1 Molecular Substances Intermolecular Forces PowerPoint Evaporation Intermolecular Forces Using the language of intermolecular forces, explain the order of the evaporation rates you observed in the first part of your experiment. The stronger these forces, the lower. (attractions between the molecules) in the liquid state have to be broken to. Explain how intermolecular forces affect rates of vaporization, evaporation, and condensation. To understand that the equilibrium vapor pressure of. Evaporation Intermolecular Forces.
From sciencenotes.org
Intermolecular Forces in Chemistry Evaporation Intermolecular Forces Explain how intermolecular forces affect rates of vaporization, evaporation, and condensation. (attractions between the molecules) in the liquid state have to be broken to. Why is evaporation an endothermic process? Using the language of intermolecular forces, explain the order of the evaporation rates you observed in the first part of your experiment. The stronger these forces, the lower. The larger. Evaporation Intermolecular Forces.
From studylib.net
Intermolecular Forces Evaporation Intermolecular Forces Explain how intermolecular forces affect rates of vaporization, evaporation, and condensation. The larger the intermolecular forces in a compound, the slower its evaporation rate. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Evaporation occurs when the probe is removed from the liquidõs container. (attractions between the molecules) in the. Evaporation Intermolecular Forces.
From www.chemicals.co.uk
What is the Definition of Evaporation in Chemistry? Evaporation Intermolecular Forces To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Evaporation occurs when the probe is removed from the liquidõs container. The larger the intermolecular forces in a compound, the slower its evaporation rate. Explain how intermolecular forces affect rates of vaporization, evaporation, and condensation. Why is evaporation an endothermic process?. Evaporation Intermolecular Forces.
From www.youtube.com
Evaporation and Intermolecular Forces YouTube Evaporation Intermolecular Forces A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Using the language of intermolecular forces, explain the order of the evaporation rates you observed in the first part of your experiment. Explain how intermolecular forces. Evaporation Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Liquids, Solids, and Intermolecular Forces PowerPoint Evaporation Intermolecular Forces This evaporation is an endothermic process that results in a temperature. In order of decreasing strength, the important. Using the language of intermolecular forces, explain the order of the evaporation rates you observed in the first part of your experiment. Explain how intermolecular forces affect rates of vaporization, evaporation, and condensation. Evaporation occurs when the probe is removed from the. Evaporation Intermolecular Forces.
From www.youtube.com
Lab Evaporation and Intermolecular Forces YouTube Evaporation Intermolecular Forces Evaporation occurs when the probe is removed from the liquidõs container. The stronger these forces, the lower. Explain how intermolecular forces affect rates of vaporization, evaporation, and condensation. The larger the intermolecular forces in a compound, the slower its evaporation rate. In order of decreasing strength, the important. A liquid’s vapor pressure is directly related to the intermolecular forces present. Evaporation Intermolecular Forces.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Evaporation Intermolecular Forces A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. This evaporation is an endothermic process that results in a temperature. Explain how intermolecular forces affect rates of vaporization, evaporation, and condensation. Using the language of intermolecular forces, explain the order of the evaporation rates you observed in the first part of your experiment. (attractions. Evaporation Intermolecular Forces.
From ctdsdcard.blogspot.com
Lab 15 Evaporation and Intermolecular Attractions Evaporation Intermolecular Forces (attractions between the molecules) in the liquid state have to be broken to. A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. Explain how intermolecular forces affect rates of vaporization, evaporation, and condensation. Evaporation occurs when the probe is removed from the liquidõs container. The larger the intermolecular forces in a compound, the slower. Evaporation Intermolecular Forces.
From www.youtube.com
Intermolecular Forces, Surface Tension, & Evaporation YouTube Evaporation Intermolecular Forces Explain how intermolecular forces affect rates of vaporization, evaporation, and condensation. Why is evaporation an endothermic process? To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. This evaporation is an endothermic process that results in a temperature. A liquid’s vapor pressure is directly related to the intermolecular forces present between. Evaporation Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Evaporation Intermolecular Forces The stronger these forces, the lower. Explain how intermolecular forces affect rates of vaporization, evaporation, and condensation. (attractions between the molecules) in the liquid state have to be broken to. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Using the language of intermolecular forces, explain the order of the. Evaporation Intermolecular Forces.
From slideplayer.com
Intermolecular forces ppt download Evaporation Intermolecular Forces Why is evaporation an endothermic process? In order of decreasing strength, the important. This evaporation is an endothermic process that results in a temperature. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Evaporation occurs when the probe is removed from the liquidõs container. Using the language of intermolecular forces,. Evaporation Intermolecular Forces.
From www.numerade.com
SOLVED Evaporation and Intermolecular Forces Based on your review of Evaporation Intermolecular Forces To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. In order of decreasing strength, the important. Why is evaporation an endothermic process? The larger the intermolecular forces in a compound, the slower its evaporation rate. (attractions between the molecules) in the liquid state have to be broken to. A liquid’s. Evaporation Intermolecular Forces.
From www.youtube.com
Intermolecular Forces Trends Melting & Boiling Point, Viscosity Evaporation Intermolecular Forces Explain how intermolecular forces affect rates of vaporization, evaporation, and condensation. Using the language of intermolecular forces, explain the order of the evaporation rates you observed in the first part of your experiment. A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. The stronger these forces, the lower. Evaporation occurs when the probe is. Evaporation Intermolecular Forces.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Evaporation Intermolecular Forces To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Evaporation occurs when the probe is removed from the liquidõs container. This evaporation is an endothermic process that results in a temperature. Explain how intermolecular forces affect rates of vaporization, evaporation, and condensation. Using the language of intermolecular forces, explain the. Evaporation Intermolecular Forces.
From www.youtube.com
12 Nust Entrance Test Rate of Evaporation, Intermolecular Forces Evaporation Intermolecular Forces A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. In order of decreasing strength, the important. This evaporation is an endothermic process that results in a temperature. (attractions between the molecules) in the liquid state have to be broken to. Using the language of intermolecular forces, explain the order of the evaporation rates you. Evaporation Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Evaporation Intermolecular Forces A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. The stronger these forces, the lower. This evaporation is an endothermic process that results in a temperature. Why is evaporation an endothermic process? The larger the intermolecular forces in a compound, the slower its evaporation rate. To understand that the equilibrium vapor pressure of a. Evaporation Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular forces Generalizing properties PowerPoint Evaporation Intermolecular Forces The stronger these forces, the lower. Using the language of intermolecular forces, explain the order of the evaporation rates you observed in the first part of your experiment. Explain how intermolecular forces affect rates of vaporization, evaporation, and condensation. Why is evaporation an endothermic process? This evaporation is an endothermic process that results in a temperature. A liquid’s vapor pressure. Evaporation Intermolecular Forces.
From studylib.net
Intermolecular Forces the four types chemistry Evaporation Intermolecular Forces A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. Using the language of intermolecular forces, explain the order of the evaporation rates you observed in the first part of your experiment. Evaporation occurs when the probe is removed from the liquidõs container. To understand that the equilibrium vapor pressure of a liquid depends on. Evaporation Intermolecular Forces.
From www.slideserve.com
PPT Chapter 12 Intermolecular Forces and Liquids PowerPoint Evaporation Intermolecular Forces The stronger these forces, the lower. Why is evaporation an endothermic process? Evaporation occurs when the probe is removed from the liquidõs container. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. This evaporation is an endothermic process that results in a temperature. Using the language of intermolecular forces, explain. Evaporation Intermolecular Forces.
From www.scribd.com
Intermolecular Forces and Liquids and Solids 2 PDF Evaporation Evaporation Intermolecular Forces The stronger these forces, the lower. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. In order of decreasing strength, the important. Why is evaporation an endothermic process? Using the language of intermolecular forces, explain the order of the evaporation rates you observed in the first part of your experiment.. Evaporation Intermolecular Forces.
From slideplayer.com
Chapter Intermolecular Forces ppt download Evaporation Intermolecular Forces A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. Explain how intermolecular forces affect rates of vaporization, evaporation, and condensation. Evaporation occurs when the probe is removed from the liquidõs container. In order of decreasing strength, the important. Using the language of intermolecular forces, explain the order of the evaporation rates you observed in. Evaporation Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces, Liquids, and Solids PowerPoint Evaporation Intermolecular Forces A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. Explain how intermolecular forces affect rates of vaporization, evaporation, and condensation. In order of decreasing strength, the important. Using the language of intermolecular forces, explain the order of the evaporation rates you observed in the first part of your experiment. Evaporation occurs when the probe. Evaporation Intermolecular Forces.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Evaporation Intermolecular Forces Why is evaporation an endothermic process? To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Evaporation occurs when the probe is removed from the liquidõs container. A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. Using the language of intermolecular forces, explain the order. Evaporation Intermolecular Forces.
From slideplayer.com
Intermolecular forces ppt download Evaporation Intermolecular Forces The larger the intermolecular forces in a compound, the slower its evaporation rate. Why is evaporation an endothermic process? This evaporation is an endothermic process that results in a temperature. The stronger these forces, the lower. In order of decreasing strength, the important. Using the language of intermolecular forces, explain the order of the evaporation rates you observed in the. Evaporation Intermolecular Forces.
From studylib.net
A. Evaporation and Vapor Pressure Evaporation Intermolecular Forces The stronger these forces, the lower. A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. (attractions between the molecules) in the liquid state have to be broken to. Evaporation occurs when the probe is removed from the liquidõs container. In order of decreasing strength, the important. To understand that the equilibrium vapor pressure of. Evaporation Intermolecular Forces.
From slidetodoc.com
Intermolecular Forces The physical properties of melting point Evaporation Intermolecular Forces Why is evaporation an endothermic process? The larger the intermolecular forces in a compound, the slower its evaporation rate. (attractions between the molecules) in the liquid state have to be broken to. Evaporation occurs when the probe is removed from the liquidõs container. This evaporation is an endothermic process that results in a temperature. To understand that the equilibrium vapor. Evaporation Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Evaporation Intermolecular Forces Evaporation occurs when the probe is removed from the liquidõs container. The larger the intermolecular forces in a compound, the slower its evaporation rate. (attractions between the molecules) in the liquid state have to be broken to. The stronger these forces, the lower. Explain how intermolecular forces affect rates of vaporization, evaporation, and condensation. A liquid’s vapor pressure is directly. Evaporation Intermolecular Forces.
From www.studocu.com
01 Evaporation and Intermolecular Forces Evaporation and Evaporation Intermolecular Forces To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. In order of decreasing strength, the important. Explain how intermolecular forces affect rates of vaporization, evaporation, and condensation. The larger the intermolecular forces in a compound, the slower its evaporation rate. A liquid’s vapor pressure is directly related to the intermolecular. Evaporation Intermolecular Forces.
From www.slideserve.com
PPT Vaporization PowerPoint Presentation, free download ID3207099 Evaporation Intermolecular Forces Using the language of intermolecular forces, explain the order of the evaporation rates you observed in the first part of your experiment. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. This evaporation is an endothermic process that results in a temperature. A liquid’s vapor pressure is directly related to. Evaporation Intermolecular Forces.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Evaporation Intermolecular Forces To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Using the language of intermolecular forces, explain the order of the evaporation rates you observed in the first part of your experiment. Why is evaporation an endothermic process? In order of decreasing strength, the important. This evaporation is an endothermic process. Evaporation Intermolecular Forces.
From www.chegg.com
Solved Evaporation and Intermolecular Forces Substance At Evaporation Intermolecular Forces This evaporation is an endothermic process that results in a temperature. Using the language of intermolecular forces, explain the order of the evaporation rates you observed in the first part of your experiment. The stronger these forces, the lower. Evaporation occurs when the probe is removed from the liquidõs container. The larger the intermolecular forces in a compound, the slower. Evaporation Intermolecular Forces.
From www.studocu.com
Intermolecular+Forces Evaporation, Temperature, and Intermolecular Evaporation Intermolecular Forces This evaporation is an endothermic process that results in a temperature. The stronger these forces, the lower. Why is evaporation an endothermic process? Using the language of intermolecular forces, explain the order of the evaporation rates you observed in the first part of your experiment. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and. Evaporation Intermolecular Forces.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Evaporation Intermolecular Forces Evaporation occurs when the probe is removed from the liquidõs container. Why is evaporation an endothermic process? This evaporation is an endothermic process that results in a temperature. Using the language of intermolecular forces, explain the order of the evaporation rates you observed in the first part of your experiment. A liquid’s vapor pressure is directly related to the intermolecular. Evaporation Intermolecular Forces.
From chemistrytalk.org
Influence of Intermolecular Forces ChemTalk Evaporation Intermolecular Forces (attractions between the molecules) in the liquid state have to be broken to. A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. Evaporation occurs when the probe is removed from the liquidõs container. The stronger these forces, the lower. Explain how intermolecular forces affect rates of vaporization, evaporation, and condensation. The larger the intermolecular. Evaporation Intermolecular Forces.