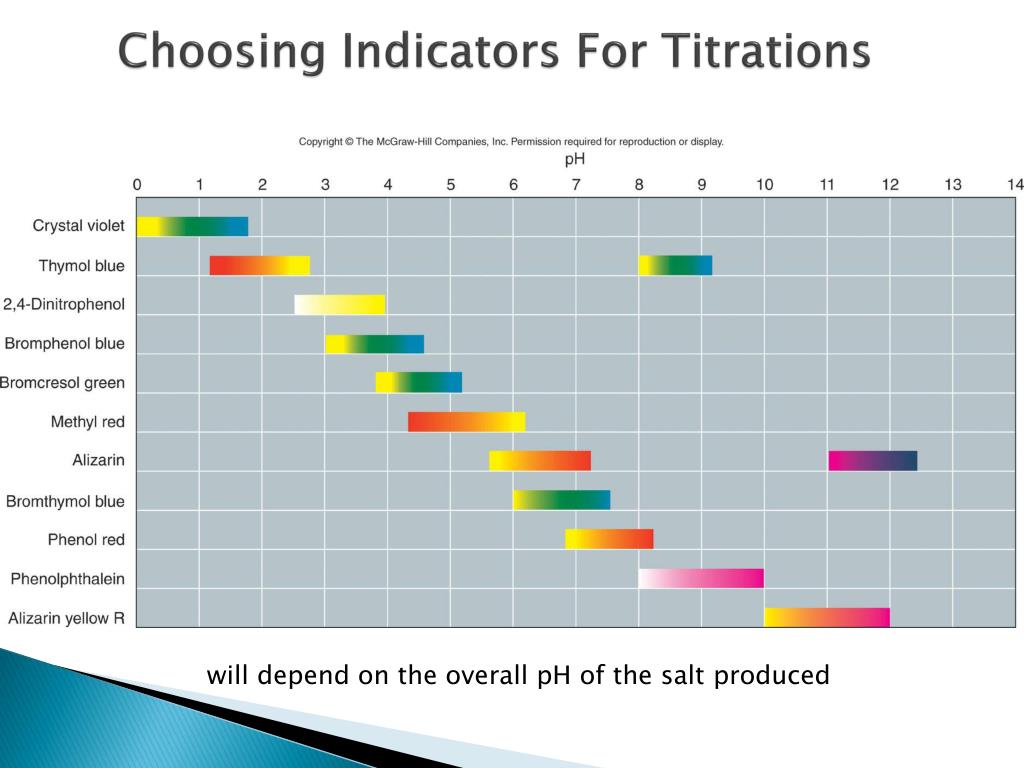
Indicators And Titration . They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. Indicators are substances whose solutions change color due to changes in ph. This page assumes that you know about ph curves for all the. The volumes of acids and alkali solutions that react with each other can be measured by titration using a. Both indicators change colour over a specific ph range. For the titration of a weak acid, however, the ph at the equivalence point is greater than 7.0, so an indicator such as phenolphthalein or thymol blue, with pkin > 7.0, should be used. The two most common indicators that are used in titrations are methyl orange and phenolphthalein.
from exordpaul.blob.core.windows.net
Both indicators change colour over a specific ph range. The volumes of acids and alkali solutions that react with each other can be measured by titration using a. For the titration of a weak acid, however, the ph at the equivalence point is greater than 7.0, so an indicator such as phenolphthalein or thymol blue, with pkin > 7.0, should be used. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Indicators are substances whose solutions change color due to changes in ph. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. This page assumes that you know about ph curves for all the.
Titration For Indicators at Robert Creech blog
Indicators And Titration For the titration of a weak acid, however, the ph at the equivalence point is greater than 7.0, so an indicator such as phenolphthalein or thymol blue, with pkin > 7.0, should be used. For the titration of a weak acid, however, the ph at the equivalence point is greater than 7.0, so an indicator such as phenolphthalein or thymol blue, with pkin > 7.0, should be used. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. This page assumes that you know about ph curves for all the. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. Indicators are substances whose solutions change color due to changes in ph. Both indicators change colour over a specific ph range. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The volumes of acids and alkali solutions that react with each other can be measured by titration using a.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Indicators And Titration The volumes of acids and alkali solutions that react with each other can be measured by titration using a. Both indicators change colour over a specific ph range. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. This page assumes that you know about ph. Indicators And Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Indicators And Titration Indicators are substances whose solutions change color due to changes in ph. This page assumes that you know about ph curves for all the. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. Both indicators change colour over a specific ph range. They. Indicators And Titration.
From courses.lumenlearning.com
AcidBase Titrations Boundless Chemistry Indicators And Titration In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. For the titration of a weak acid, however, the ph. Indicators And Titration.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID9234620 Indicators And Titration In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. This page assumes that you know about ph curves for all the. Indicators are substances whose solutions change color due to changes in ph. For the titration of a weak acid, however, the ph. Indicators And Titration.
From exordpaul.blob.core.windows.net
Titration For Indicators at Robert Creech blog Indicators And Titration They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. Both indicators change colour over a specific ph range. For. Indicators And Titration.
From www.science-revision.co.uk
Titrations Indicators And Titration They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. For the titration of a weak acid, however, the ph at the equivalence point is greater than 7.0, so an indicator such as phenolphthalein or thymol blue, with pkin > 7.0, should be used. Indicators are. Indicators And Titration.
From dxonbtcto.blob.core.windows.net
Chemical Indicator AcidBase Titration at Hilda Johnston blog Indicators And Titration They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Indicators are substances whose solutions change color due to changes in ph. The volumes of acids and alkali solutions that react. Indicators And Titration.
From cezsemit.blob.core.windows.net
Why Are Different Indicators Used In Titration at Ladonna Anderson blog Indicators And Titration The volumes of acids and alkali solutions that react with each other can be measured by titration using a. For the titration of a weak acid, however, the ph at the equivalence point is greater than 7.0, so an indicator such as phenolphthalein or thymol blue, with pkin > 7.0, should be used. In general, for titrations of strong acids. Indicators And Titration.
From mungfali.com
Acid Base Titration Indicator Indicators And Titration They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The volumes of acids and alkali solutions that react with each other can be measured by titration using a. Both indicators. Indicators And Titration.
From wisc.pb.unizin.org
D30.1 AcidBase Indicators Chemistry 109 Fall 2021 Indicators And Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. The volumes of acids and alkali solutions that react with each other can be measured by titration using a.. Indicators And Titration.
From fphoto.photoshelter.com
science chemistry titration phenolphthalein Fundamental Photographs Indicators And Titration The volumes of acids and alkali solutions that react with each other can be measured by titration using a. This page assumes that you know about ph curves for all the. Indicators are substances whose solutions change color due to changes in ph. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. In general,. Indicators And Titration.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Indicators And Titration This page assumes that you know about ph curves for all the. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their. Indicators And Titration.
From chrominfo.blogspot.com
Chrominfo Indicators of complexometric titration Indicators And Titration Both indicators change colour over a specific ph range. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. For. Indicators And Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Indicators And Titration They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. The volumes of acids and alkali solutions that react with each other can be measured by titration using a. Indicators are substances whose solutions change color due to changes in ph. The two most common indicators. Indicators And Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Indicators And Titration The volumes of acids and alkali solutions that react with each other can be measured by titration using a. This page assumes that you know about ph curves for all the. For the titration of a weak acid, however, the ph at the equivalence point is greater than 7.0, so an indicator such as phenolphthalein or thymol blue, with pkin. Indicators And Titration.
From exoraquci.blob.core.windows.net
List Of Indicators Used In Titration at Jennifer Bowman blog Indicators And Titration In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. The volumes of acids and alkali solutions that react with. Indicators And Titration.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector Indicators And Titration This page assumes that you know about ph curves for all the. Both indicators change colour over a specific ph range. Indicators are substances whose solutions change color due to changes in ph. For the titration of a weak acid, however, the ph at the equivalence point is greater than 7.0, so an indicator such as phenolphthalein or thymol blue,. Indicators And Titration.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Indicators And Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Both indicators change colour over a specific ph range. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. For the titration of a weak acid, however, the ph. Indicators And Titration.
From www.youtube.com
Acids and Bases Choosing an Indicator for Titration YouTube Indicators And Titration In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. For the titration of a weak acid, however, the ph at the equivalence point is greater than 7.0, so. Indicators And Titration.
From www.slideshare.net
Acid base titration Indicators And Titration The volumes of acids and alkali solutions that react with each other can be measured by titration using a. For the titration of a weak acid, however, the ph at the equivalence point is greater than 7.0, so an indicator such as phenolphthalein or thymol blue, with pkin > 7.0, should be used. Both indicators change colour over a specific. Indicators And Titration.
From www.alamy.com
Phenolphthalein is used as a single indicator in acidbase titrations Indicators And Titration For the titration of a weak acid, however, the ph at the equivalence point is greater than 7.0, so an indicator such as phenolphthalein or thymol blue, with pkin > 7.0, should be used. This page assumes that you know about ph curves for all the. The two most common indicators that are used in titrations are methyl orange and. Indicators And Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Indicators And Titration For the titration of a weak acid, however, the ph at the equivalence point is greater than 7.0, so an indicator such as phenolphthalein or thymol blue, with pkin > 7.0, should be used. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. In general,. Indicators And Titration.
From freesvg.org
Redox Titration Using Indicator Free SVG Indicators And Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Both indicators change colour over a specific ph range. Indicators are substances whose solutions change color due to changes in ph. This page assumes that you know about ph curves for all the. In general, for titrations of strong acids with strong bases (and vice. Indicators And Titration.
From exocowbmz.blob.core.windows.net
Indicator Used In Calcium Titration at Hattie Murphy blog Indicators And Titration They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Indicators are substances whose solutions change color due to changes in ph. Both indicators change colour over a specific ph range. In general, for titrations of strong acids with strong bases (and vice versa), any indicator. Indicators And Titration.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Indicators And Titration Indicators are substances whose solutions change color due to changes in ph. For the titration of a weak acid, however, the ph at the equivalence point is greater than 7.0, so an indicator such as phenolphthalein or thymol blue, with pkin > 7.0, should be used. The volumes of acids and alkali solutions that react with each other can be. Indicators And Titration.
From cezsemit.blob.core.windows.net
Why Are Different Indicators Used In Titration at Ladonna Anderson blog Indicators And Titration They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. The volumes of acids and alkali solutions that react with each other can be measured by titration using a. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. This page. Indicators And Titration.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Indicators And Titration In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. Indicators are substances whose solutions change color due to changes in ph. This page assumes that you know about ph curves for all the. The volumes of acids and alkali solutions that react with. Indicators And Titration.
From psu.pb.unizin.org
AcidBase Titrations (14.7) Chemistry 112 (Chapters 1217 of OpenStax Indicators And Titration For the titration of a weak acid, however, the ph at the equivalence point is greater than 7.0, so an indicator such as phenolphthalein or thymol blue, with pkin > 7.0, should be used. Indicators are substances whose solutions change color due to changes in ph. In general, for titrations of strong acids with strong bases (and vice versa), any. Indicators And Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Indicators And Titration Indicators are substances whose solutions change color due to changes in ph. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their. Indicators And Titration.
From www.microlit.com
An Advanced Guide to Titration Microlit Indicators And Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. This page assumes that you know about ph curves for all the. The volumes of acids and alkali solutions that react. Indicators And Titration.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Indicators And Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases,. Indicators And Titration.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations (GCSE) — the science hive Indicators And Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Indicators are substances whose solutions change color due to changes in ph. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. Both indicators change colour over a specific. Indicators And Titration.
From chem.libretexts.org
Redox Titration Chemistry LibreTexts Indicators And Titration Both indicators change colour over a specific ph range. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. Indicators. Indicators And Titration.
From stock.adobe.com
Acidbase titration and phenolphthalein indicator Stock Vector Adobe Indicators And Titration Indicators are substances whose solutions change color due to changes in ph. For the titration of a weak acid, however, the ph at the equivalence point is greater than 7.0, so an indicator such as phenolphthalein or thymol blue, with pkin > 7.0, should be used. In general, for titrations of strong acids with strong bases (and vice versa), any. Indicators And Titration.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Indicators And Titration Both indicators change colour over a specific ph range. The volumes of acids and alkali solutions that react with each other can be measured by titration using a. This page assumes that you know about ph curves for all the. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences. Indicators And Titration.