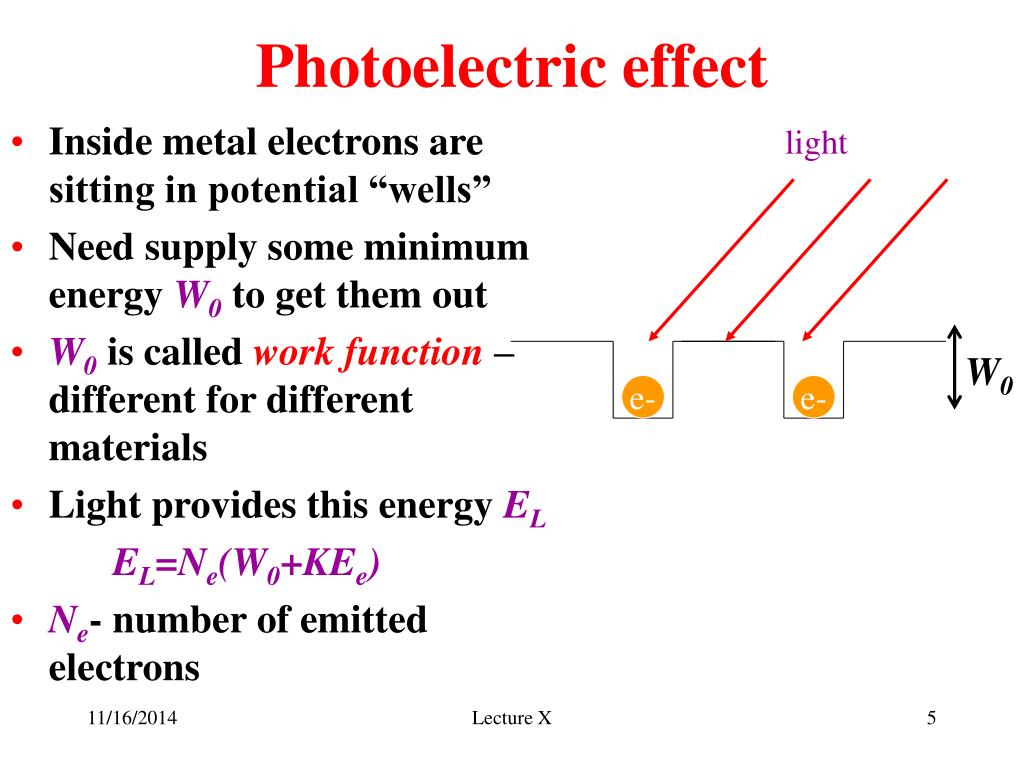
Photoelectric Effect Units . Einstein proposed photons to be quanta of em radiation having energy \(e=h f\), where \(f\) is the frequency of the radiation. In simple terms, when light shines on a material, it can transfer its energy to electrons within the material. The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s surface. The photoelectric effect is the process in which em radiation ejects electrons from a material. Photons of frequencies above the threshold frequency will have more energy than just the work function. The energy of a photon is given as: The photoelectric effect is the process in which electromagnetic radiation ejects electrons from a material. The ejected electrons were called photoelectrons to link them to this specific behavior, which was dubbed the photoelectric effect. If this energy is sufficient, the electrons can overcome the. An amount of energy equal to the work function is used to release the photoelectron from the metal. Einstein and millikan described the photoelectric effect using a formula (in contemporary notation) that relates the maximum kinetic energy (k max). Einstein proposed photons to be quanta. When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a.
from www.slideserve.com
Photons of frequencies above the threshold frequency will have more energy than just the work function. The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s surface. The ejected electrons were called photoelectrons to link them to this specific behavior, which was dubbed the photoelectric effect. Einstein proposed photons to be quanta. Einstein proposed photons to be quanta of em radiation having energy \(e=h f\), where \(f\) is the frequency of the radiation. The photoelectric effect is the process in which em radiation ejects electrons from a material. In simple terms, when light shines on a material, it can transfer its energy to electrons within the material. The energy of a photon is given as: The photoelectric effect is the process in which electromagnetic radiation ejects electrons from a material. If this energy is sufficient, the electrons can overcome the.
PPT Photoelectric effect, photons PowerPoint Presentation, free
Photoelectric Effect Units The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s surface. In simple terms, when light shines on a material, it can transfer its energy to electrons within the material. Einstein and millikan described the photoelectric effect using a formula (in contemporary notation) that relates the maximum kinetic energy (k max). If this energy is sufficient, the electrons can overcome the. The ejected electrons were called photoelectrons to link them to this specific behavior, which was dubbed the photoelectric effect. Photons of frequencies above the threshold frequency will have more energy than just the work function. An amount of energy equal to the work function is used to release the photoelectron from the metal. The photoelectric effect is the process in which em radiation ejects electrons from a material. Einstein proposed photons to be quanta of em radiation having energy \(e=h f\), where \(f\) is the frequency of the radiation. The photoelectric effect is the process in which electromagnetic radiation ejects electrons from a material. The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s surface. Einstein proposed photons to be quanta. The energy of a photon is given as: When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a.
From www.youtube.com
Unit 3.12 Properties of Photons (Photoelectric Effect) YouTube Photoelectric Effect Units When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a. The ejected electrons were called photoelectrons to link them to this specific behavior, which was dubbed the photoelectric effect. Einstein proposed photons to be quanta of em radiation having energy \(e=h f\), where \(f\) is the frequency of the radiation. Einstein. Photoelectric Effect Units.
From www.slideserve.com
PPT Photoelectric effect, photons PowerPoint Presentation, free Photoelectric Effect Units If this energy is sufficient, the electrons can overcome the. Photons of frequencies above the threshold frequency will have more energy than just the work function. Einstein and millikan described the photoelectric effect using a formula (in contemporary notation) that relates the maximum kinetic energy (k max). An amount of energy equal to the work function is used to release. Photoelectric Effect Units.
From thescienceandmathszone.com
The Photoelectric Effect, Photons and Planck’s Equation The Science Photoelectric Effect Units An amount of energy equal to the work function is used to release the photoelectron from the metal. If this energy is sufficient, the electrons can overcome the. The photoelectric effect is the process in which em radiation ejects electrons from a material. When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently,. Photoelectric Effect Units.
From www.researchgate.net
Schematic representation of the photoelectric effect and Compton Photoelectric Effect Units An amount of energy equal to the work function is used to release the photoelectron from the metal. The photoelectric effect is the process in which em radiation ejects electrons from a material. The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s surface. Photons of. Photoelectric Effect Units.
From thescienceandmathszone.com
The Photoelectric Effect, Photons and Planck’s Equation The Science Photoelectric Effect Units The energy of a photon is given as: Einstein and millikan described the photoelectric effect using a formula (in contemporary notation) that relates the maximum kinetic energy (k max). If this energy is sufficient, the electrons can overcome the. Einstein proposed photons to be quanta. The photoelectric effect is the process in which electromagnetic radiation ejects electrons from a material.. Photoelectric Effect Units.
From slideplayer.com
The Photoelectric Effect ppt download Photoelectric Effect Units In simple terms, when light shines on a material, it can transfer its energy to electrons within the material. Einstein proposed photons to be quanta. An amount of energy equal to the work function is used to release the photoelectron from the metal. When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently,. Photoelectric Effect Units.
From www.physicstutoronline.co.uk
Photoelectric Effect explained in this fully illustrated article Photoelectric Effect Units An amount of energy equal to the work function is used to release the photoelectron from the metal. The energy of a photon is given as: Einstein proposed photons to be quanta of em radiation having energy \(e=h f\), where \(f\) is the frequency of the radiation. The photoelectric effect is the process in which em radiation ejects electrons from. Photoelectric Effect Units.
From www.physicstutoronline.co.uk
Photoelectric Effect explained in this fully illustrated article Photoelectric Effect Units If this energy is sufficient, the electrons can overcome the. When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a. The ejected electrons were called photoelectrons to link them to this specific behavior, which was dubbed the photoelectric effect. The photoelectric effect is the process in which electromagnetic radiation ejects electrons. Photoelectric Effect Units.
From physics.icalculator.com
Laws of the Photoelectric Effect iCalculator™ Photoelectric Effect Units The energy of a photon is given as: Einstein proposed photons to be quanta of em radiation having energy \(e=h f\), where \(f\) is the frequency of the radiation. An amount of energy equal to the work function is used to release the photoelectron from the metal. Einstein proposed photons to be quanta. When a metal surface is exposed to. Photoelectric Effect Units.
From www.scribd.com
Photoelectric Effect Presentation PDF Photoelectric Effect Photoelectric Effect Units The photoelectric effect is the process in which em radiation ejects electrons from a material. Einstein proposed photons to be quanta. The ejected electrons were called photoelectrons to link them to this specific behavior, which was dubbed the photoelectric effect. Einstein and millikan described the photoelectric effect using a formula (in contemporary notation) that relates the maximum kinetic energy (k. Photoelectric Effect Units.
From www.youtube.com
Photoelectric effect animation Explained with 3d YouTube Photoelectric Effect Units The ejected electrons were called photoelectrons to link them to this specific behavior, which was dubbed the photoelectric effect. Einstein proposed photons to be quanta. The photoelectric effect is the process in which em radiation ejects electrons from a material. In simple terms, when light shines on a material, it can transfer its energy to electrons within the material. Einstein. Photoelectric Effect Units.
From www.studypool.com
SOLUTION Concept map of unit photoelectric effect class12 subject Photoelectric Effect Units In simple terms, when light shines on a material, it can transfer its energy to electrons within the material. Einstein proposed photons to be quanta. An amount of energy equal to the work function is used to release the photoelectron from the metal. The ejected electrons were called photoelectrons to link them to this specific behavior, which was dubbed the. Photoelectric Effect Units.
From www.electricity-magnetism.org
Photoelectric Effect Definition & Mechanism Electricity Photoelectric Effect Units When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a. In simple terms, when light shines on a material, it can transfer its energy to electrons within the material. Einstein proposed photons to be quanta of em radiation having energy \(e=h f\), where \(f\) is the frequency of the radiation. If. Photoelectric Effect Units.
From www.slideserve.com
PPT Photoelectric Effect PowerPoint Presentation, free download ID Photoelectric Effect Units Einstein proposed photons to be quanta of em radiation having energy \(e=h f\), where \(f\) is the frequency of the radiation. Photons of frequencies above the threshold frequency will have more energy than just the work function. The energy of a photon is given as: In simple terms, when light shines on a material, it can transfer its energy to. Photoelectric Effect Units.
From www.nsta.org
The Photoelectric Effect NSTA Photoelectric Effect Units The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s surface. The photoelectric effect is the process in which electromagnetic radiation ejects electrons from a material. Einstein and millikan described the photoelectric effect using a formula (in contemporary notation) that relates the maximum kinetic energy (k. Photoelectric Effect Units.
From www.slideserve.com
PPT Photoelectric effect, photons PowerPoint Presentation, free Photoelectric Effect Units When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a. In simple terms, when light shines on a material, it can transfer its energy to electrons within the material. Einstein and millikan described the photoelectric effect using a formula (in contemporary notation) that relates the maximum kinetic energy (k max). Photons. Photoelectric Effect Units.
From www.researchgate.net
Schematic representation of the photoelectric effect. Download Photoelectric Effect Units If this energy is sufficient, the electrons can overcome the. In simple terms, when light shines on a material, it can transfer its energy to electrons within the material. The photoelectric effect is the process in which em radiation ejects electrons from a material. Einstein and millikan described the photoelectric effect using a formula (in contemporary notation) that relates the. Photoelectric Effect Units.
From www.youtube.com
Einstein's Photoelectric Equation YouTube Photoelectric Effect Units The energy of a photon is given as: The ejected electrons were called photoelectrons to link them to this specific behavior, which was dubbed the photoelectric effect. In simple terms, when light shines on a material, it can transfer its energy to electrons within the material. The photoelectric effect refers to the phenomenon where light, typically in the form of. Photoelectric Effect Units.
From scientificimaging.com
C3) The Photoelectric Effect in Image Sensors Scientific Imaging, Inc. Photoelectric Effect Units The photoelectric effect is the process in which electromagnetic radiation ejects electrons from a material. The ejected electrons were called photoelectrons to link them to this specific behavior, which was dubbed the photoelectric effect. Photons of frequencies above the threshold frequency will have more energy than just the work function. When a metal surface is exposed to a monochromatic electromagnetic. Photoelectric Effect Units.
From www.youtube.com
The Photoelectric Effect YouTube Photoelectric Effect Units The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s surface. Einstein and millikan described the photoelectric effect using a formula (in contemporary notation) that relates the maximum kinetic energy (k max). An amount of energy equal to the work function is used to release the. Photoelectric Effect Units.
From general.chemistrysteps.com
Photoelectric Effect Chemistry Steps Photoelectric Effect Units An amount of energy equal to the work function is used to release the photoelectron from the metal. When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a. In simple terms, when light shines on a material, it can transfer its energy to electrons within the material. Photons of frequencies above. Photoelectric Effect Units.
From www.slideserve.com
PPT Photoelectric effect, photons PowerPoint Presentation, free Photoelectric Effect Units The photoelectric effect is the process in which electromagnetic radiation ejects electrons from a material. Einstein proposed photons to be quanta. When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a. The ejected electrons were called photoelectrons to link them to this specific behavior, which was dubbed the photoelectric effect. An. Photoelectric Effect Units.
From www.slideserve.com
PPT The Photoelectric Effect PowerPoint Presentation, free download Photoelectric Effect Units If this energy is sufficient, the electrons can overcome the. When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a. In simple terms, when light shines on a material, it can transfer its energy to electrons within the material. The photoelectric effect is the process in which electromagnetic radiation ejects electrons. Photoelectric Effect Units.
From syzygyastro.hubpages.com
How the Physics of can Generate Electricity HubPages Photoelectric Effect Units The energy of a photon is given as: Einstein proposed photons to be quanta. In simple terms, when light shines on a material, it can transfer its energy to electrons within the material. Einstein and millikan described the photoelectric effect using a formula (in contemporary notation) that relates the maximum kinetic energy (k max). The photoelectric effect refers to the. Photoelectric Effect Units.
From www.dreamstime.com
Photoelectric Effect Infographic Diagram Electrochemistry Stock Vector Photoelectric Effect Units The photoelectric effect is the process in which em radiation ejects electrons from a material. In simple terms, when light shines on a material, it can transfer its energy to electrons within the material. The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s surface. Einstein. Photoelectric Effect Units.
From www.scienceabc.com
What Is The Photoelectric Effect? » ScienceABC Photoelectric Effect Units Einstein proposed photons to be quanta. If this energy is sufficient, the electrons can overcome the. In simple terms, when light shines on a material, it can transfer its energy to electrons within the material. The photoelectric effect is the process in which electromagnetic radiation ejects electrons from a material. The photoelectric effect is the process in which em radiation. Photoelectric Effect Units.
From byjus.com
Einstein's Explanation Of Photoelectric Effect Threshold Frequency Photoelectric Effect Units An amount of energy equal to the work function is used to release the photoelectron from the metal. Photons of frequencies above the threshold frequency will have more energy than just the work function. The energy of a photon is given as: If this energy is sufficient, the electrons can overcome the. The ejected electrons were called photoelectrons to link. Photoelectric Effect Units.
From general.chemistrysteps.com
Photoelectric Effect Chemistry Steps Photoelectric Effect Units In simple terms, when light shines on a material, it can transfer its energy to electrons within the material. Einstein proposed photons to be quanta of em radiation having energy \(e=h f\), where \(f\) is the frequency of the radiation. The photoelectric effect is the process in which electromagnetic radiation ejects electrons from a material. The photoelectric effect is the. Photoelectric Effect Units.
From www.livescience.com
Photoelectric Effect Explanation & Applications Live Science Photoelectric Effect Units When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a. Einstein proposed photons to be quanta of em radiation having energy \(e=h f\), where \(f\) is the frequency of the radiation. Einstein proposed photons to be quanta. An amount of energy equal to the work function is used to release the. Photoelectric Effect Units.
From www.youtube.com
Photoelectric effect graph and spectrum As Physics Unit 2 lesson 8 part Photoelectric Effect Units Einstein proposed photons to be quanta of em radiation having energy \(e=h f\), where \(f\) is the frequency of the radiation. When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a. The energy of a photon is given as: Einstein and millikan described the photoelectric effect using a formula (in contemporary. Photoelectric Effect Units.
From www.youtube.com
Einstein's explanation of the Photoelectric Effect YouTube Photoelectric Effect Units If this energy is sufficient, the electrons can overcome the. Einstein proposed photons to be quanta of em radiation having energy \(e=h f\), where \(f\) is the frequency of the radiation. The photoelectric effect is the process in which em radiation ejects electrons from a material. The photoelectric effect refers to the phenomenon where light, typically in the form of. Photoelectric Effect Units.
From www.nsta.org
The Photoelectric Effect NSTA Photoelectric Effect Units The energy of a photon is given as: The ejected electrons were called photoelectrons to link them to this specific behavior, which was dubbed the photoelectric effect. Einstein proposed photons to be quanta of em radiation having energy \(e=h f\), where \(f\) is the frequency of the radiation. Einstein proposed photons to be quanta. An amount of energy equal to. Photoelectric Effect Units.
From www.radiation-dosimetry.org
What is Photoelectric Effect Definition Photoelectric Effect Units Einstein proposed photons to be quanta. Photons of frequencies above the threshold frequency will have more energy than just the work function. When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a. The photoelectric effect is the process in which electromagnetic radiation ejects electrons from a material. The photoelectric effect refers. Photoelectric Effect Units.
From www.youtube.com
What is Photoelectric Effect? Laws and Einstein's Phototelectric Photoelectric Effect Units Einstein proposed photons to be quanta. The ejected electrons were called photoelectrons to link them to this specific behavior, which was dubbed the photoelectric effect. Einstein and millikan described the photoelectric effect using a formula (in contemporary notation) that relates the maximum kinetic energy (k max). In simple terms, when light shines on a material, it can transfer its energy. Photoelectric Effect Units.
From www.slideserve.com
PPT 1. Photoelectric effect PowerPoint Presentation, free download Photoelectric Effect Units If this energy is sufficient, the electrons can overcome the. When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a. The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s surface. The photoelectric effect is the process. Photoelectric Effect Units.