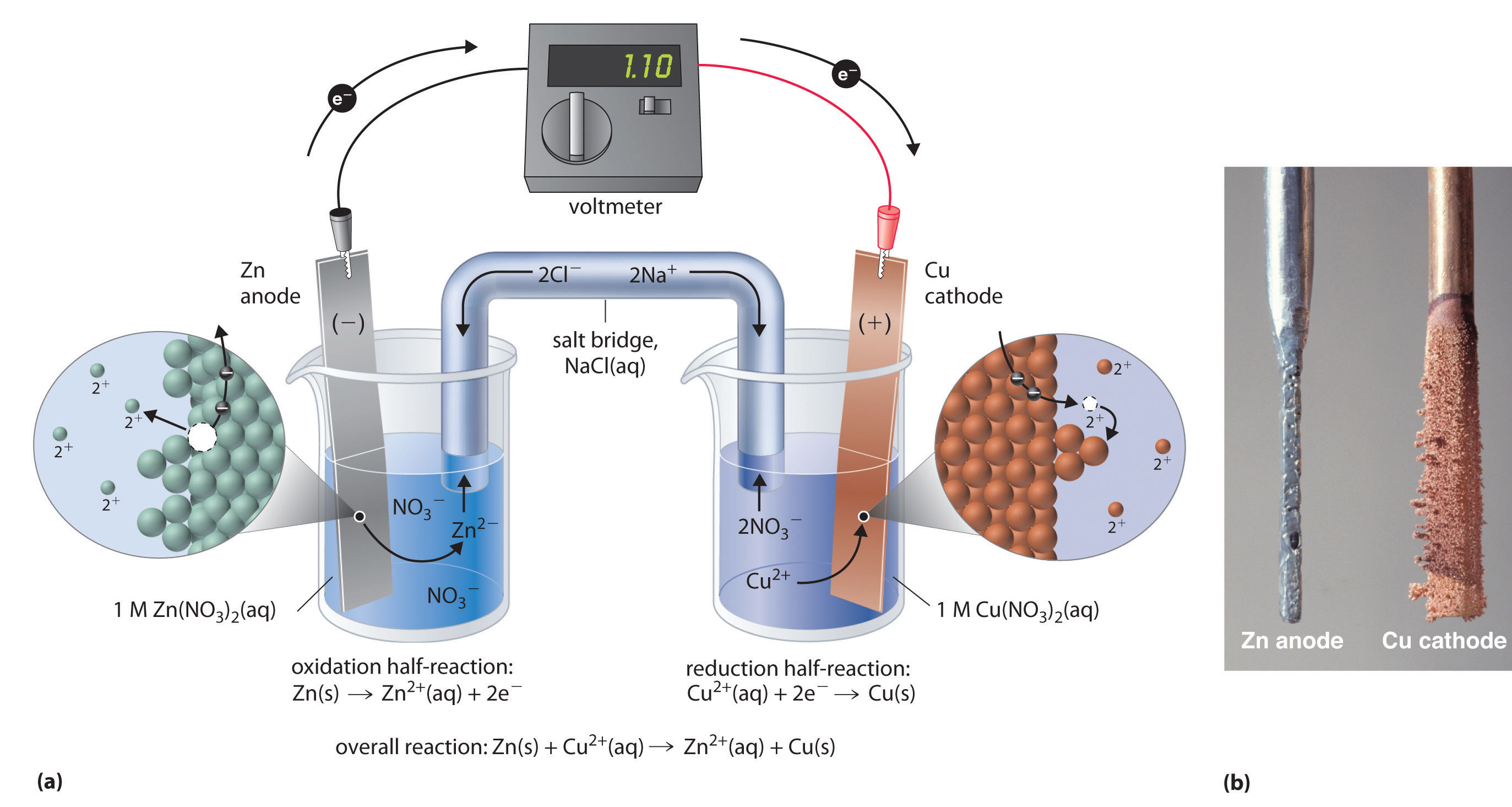
Zinc Chloride Cell . the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; The carbon is added to the cathode to increase.
from saylordotorg.github.io
the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; The carbon is added to the cathode to increase. the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry.
Electrochemistry
Zinc Chloride Cell the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; The carbon is added to the cathode to increase.
From paramountchemicals.com.au
Zinc Chloride Paramount Chemicals Zinc Chloride Cell the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. The carbon is added to the cathode to increase. Zinc Chloride Cell.
From www.pakistanchemical.com
Zinc Chloride PAKISTAN CHEMICAL Zinc Chloride Cell the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; The carbon is added to the cathode to increase. Zinc Chloride Cell.
From www.researchgate.net
Electrochemical reversible cell containing silver and zinc in Zinc Chloride Cell the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. The carbon is added to the cathode to increase. Zinc Chloride Cell.
From www.alibaba.com
Silver Zinc Chloride Buy Silver Zinc Chloride,Zinc Chloride (battery Zinc Chloride Cell the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. The carbon is added to the cathode to increase. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; Zinc Chloride Cell.
From www.electricity-magnetism.org
Zincchloride battery extraheavyduty Electricity Zinc Chloride Cell The carbon is added to the cathode to increase. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. Zinc Chloride Cell.
From www.linkedin.com
Applications of Zinc Chloride Zinc Chloride Cell the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. The carbon is added to the cathode to increase. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; Zinc Chloride Cell.
From hxecmkzrl.blob.core.windows.net
Zinc Chloride Vs Alkaline at Kathryn Walker blog Zinc Chloride Cell the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. The carbon is added to the cathode to increase. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; Zinc Chloride Cell.
From saylordotorg.github.io
Electrochemistry Zinc Chloride Cell the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; The carbon is added to the cathode to increase. Zinc Chloride Cell.
From hxeqbamfu.blob.core.windows.net
Zinc Chloride Battery at Dewey Menard blog Zinc Chloride Cell the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; The carbon is added to the cathode to increase. Zinc Chloride Cell.
From www.researchgate.net
(PDF) Zinc Chloride Enhances the Antioxidant Status, Improving the Zinc Chloride Cell The carbon is added to the cathode to increase. the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; Zinc Chloride Cell.
From hxeqbamfu.blob.core.windows.net
Zinc Chloride Battery at Dewey Menard blog Zinc Chloride Cell the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; The carbon is added to the cathode to increase. Zinc Chloride Cell.
From studylib.net
Leclanche´ and Zinc Chloride Cell Systems Zinc Chloride Cell the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. The carbon is added to the cathode to increase. Zinc Chloride Cell.
From www.semanticscholar.org
[PDF] ZINCCARBON BATTERIES ( Leclanche ́ and Zinc Chloride Cell Zinc Chloride Cell the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; The carbon is added to the cathode to increase. Zinc Chloride Cell.
From www.911metallurgist.com
Zinc Electrowinning from Chloride Electrolyte Zinc Chloride Cell the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. The carbon is added to the cathode to increase. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; Zinc Chloride Cell.
From www.rapidonline.com
Zinc Chloride Cell AAA battery Rapid Online Zinc Chloride Cell the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. The carbon is added to the cathode to increase. Zinc Chloride Cell.
From www.britannica.com
Cell Miniaturization, Microelectronics, Components Britannica Zinc Chloride Cell The carbon is added to the cathode to increase. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. Zinc Chloride Cell.
From www.semanticscholar.org
Figure 1 from ZINCCARBON BATTERIES ( Leclanche ́ and Zinc Chloride Zinc Chloride Cell The carbon is added to the cathode to increase. the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; Zinc Chloride Cell.
From www.youtube.com
How to Write the Formula for Zinc chloride (ZnCl2) YouTube Zinc Chloride Cell the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. The carbon is added to the cathode to increase. Zinc Chloride Cell.
From www.abmole.com
Fast Red Violet LB Zinc chloride CAS 32348815 (free base) AbMole Zinc Chloride Cell the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. The carbon is added to the cathode to increase. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; Zinc Chloride Cell.
From www.electricity-magnetism.org
Advantages and Disadvantages of Zincchloride Batteries Electricity Zinc Chloride Cell the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. The carbon is added to the cathode to increase. Zinc Chloride Cell.
From www.rapidonline.com
GP GPPCC24UC004 Zinc Chloride Cell AAA battery (Pack 2) Rapid Online Zinc Chloride Cell The carbon is added to the cathode to increase. the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; Zinc Chloride Cell.
From psu.pb.unizin.org
17.3 Standard Reduction Potentials Chemistry 112 Chapters 1217 of Zinc Chloride Cell The carbon is added to the cathode to increase. the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; Zinc Chloride Cell.
From courses.lumenlearning.com
Electrolysis Chemistry for Majors Zinc Chloride Cell The carbon is added to the cathode to increase. the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; Zinc Chloride Cell.
From pubs.rsc.org
The electrolysis of fused zinc chloride in cells heated externally Zinc Chloride Cell the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. The carbon is added to the cathode to increase. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; Zinc Chloride Cell.
From www.alamy.com
Leclanche's dry cell and its later variations, zinc chloride Zinc Chloride Cell the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; The carbon is added to the cathode to increase. the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. Zinc Chloride Cell.
From www.alamy.com
Modern version of the Leclanche cell. This heavyduty zinccarbon Zinc Chloride Cell the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. The carbon is added to the cathode to increase. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; Zinc Chloride Cell.
From knowledge.electrochem.org
Electrochemistry Encyclopedia Nonrechargeable batteries Zinc Chloride Cell the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; The carbon is added to the cathode to increase. Zinc Chloride Cell.
From www.researchgate.net
Zinc chloride treatment inhibits in vitro cell proliferation in Zinc Chloride Cell The carbon is added to the cathode to increase. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. Zinc Chloride Cell.
From www.flinnsci.com
Flinn Chemicals, Zinc Chloride Zinc Chloride Cell the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; The carbon is added to the cathode to increase. the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. Zinc Chloride Cell.
From www.indiamart.com
Brefresh Chlorhexidine Gluconate Sodium Fluoride Zinc Chloride Mouth Zinc Chloride Cell the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; The carbon is added to the cathode to increase. the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. Zinc Chloride Cell.
From www.slideserve.com
PPT Electrochemical Cells (voltaic cells) PowerPoint Presentation Zinc Chloride Cell the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; The carbon is added to the cathode to increase. Zinc Chloride Cell.
From www.rapidonline.com
GP GPPCC15KC005 Zinc Chloride Cell AA Battery (Pack 2) Rapid Online Zinc Chloride Cell The carbon is added to the cathode to increase. the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; Zinc Chloride Cell.
From www.indiamart.com
Zinc Chloride at best price in Vadodara by Canton Laboratories Private Zinc Chloride Cell the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; The carbon is added to the cathode to increase. Zinc Chloride Cell.
From webmis.highland.cc.il.us
Electrolysis Zinc Chloride Cell the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; The carbon is added to the cathode to increase. Zinc Chloride Cell.
From hxeehncbp.blob.core.windows.net
Are Alkaline Batteries The Same As Carbon Zinc at Betty Sesco blog Zinc Chloride Cell the zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; The carbon is added to the cathode to increase. the original cell consisted of a solid zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry. Zinc Chloride Cell.