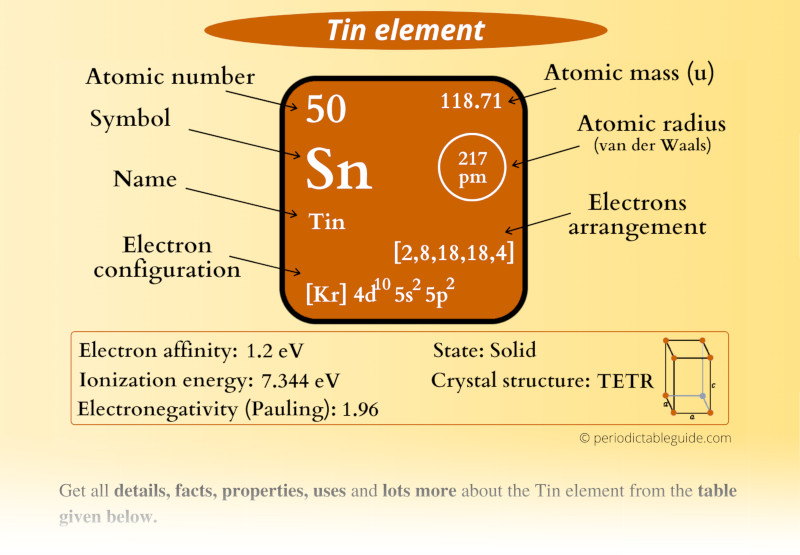
Tin Atomic Mass . The atomic mass is the mass of an atom. Tin is a silvery malleable metallic element with atomic number 50 and atomic weight 118.71. Learn about its physical and chemical properties,. Cosmically, tin is a product of neutron absorption. Tin is primarily obtained from the mineral cassiterite (sno 2) and is extracted by roasting cassiterite in a furnace with carbon. Tin is a soft, pliable metal with atomic number 50 and relative atomic mass 118.710. Its richness in stable isotopes is noteworthy. It has four valence electrons, three. In the cosmos there are 1.33 atoms of tin per 1 × 10 6 atoms of silicon, an abundance roughly equal to that of niobium, ruthenium, neodymium, or platinum. The atomic mass or relative isotopic mass refers to the mass. Find out how tin is obtained, used and its chemical behavior. Tin makes up only about 0.001% of the earth's crust and is chiefly mined in. Atomic mass of tin is 118.71 u. It has many uses, such as coating other metals, making.
from periodictableguide.com
Tin makes up only about 0.001% of the earth's crust and is chiefly mined in. The atomic mass or relative isotopic mass refers to the mass. It has many uses, such as coating other metals, making. Cosmically, tin is a product of neutron absorption. In the cosmos there are 1.33 atoms of tin per 1 × 10 6 atoms of silicon, an abundance roughly equal to that of niobium, ruthenium, neodymium, or platinum. Tin is a silvery malleable metallic element with atomic number 50 and atomic weight 118.71. Atomic mass of tin is 118.71 u. Tin is a soft, pliable metal with atomic number 50 and relative atomic mass 118.710. Learn about its physical and chemical properties,. Find out how tin is obtained, used and its chemical behavior.
Tin (Sn) Periodic Table (Element Information & More)
Tin Atomic Mass Find out how tin is obtained, used and its chemical behavior. Tin makes up only about 0.001% of the earth's crust and is chiefly mined in. It has four valence electrons, three. In the cosmos there are 1.33 atoms of tin per 1 × 10 6 atoms of silicon, an abundance roughly equal to that of niobium, ruthenium, neodymium, or platinum. The atomic mass is the mass of an atom. Tin is primarily obtained from the mineral cassiterite (sno 2) and is extracted by roasting cassiterite in a furnace with carbon. Find out how tin is obtained, used and its chemical behavior. Learn about its physical and chemical properties,. Atomic mass of tin is 118.71 u. Tin is a soft, pliable metal with atomic number 50 and relative atomic mass 118.710. Tin is a silvery malleable metallic element with atomic number 50 and atomic weight 118.71. It has many uses, such as coating other metals, making. The atomic mass or relative isotopic mass refers to the mass. Its richness in stable isotopes is noteworthy. Cosmically, tin is a product of neutron absorption.
From utedzz.blogspot.com
Periodic Table Tin Sn Periodic Table Timeline Tin Atomic Mass The atomic mass or relative isotopic mass refers to the mass. In the cosmos there are 1.33 atoms of tin per 1 × 10 6 atoms of silicon, an abundance roughly equal to that of niobium, ruthenium, neodymium, or platinum. Find out how tin is obtained, used and its chemical behavior. It has many uses, such as coating other metals,. Tin Atomic Mass.
From www.dreamstime.com
Tin Atom, with Mass and Energy Levels. Stock Vector Illustration of formula, periodic 256806989 Tin Atomic Mass Its richness in stable isotopes is noteworthy. The atomic mass is the mass of an atom. Tin makes up only about 0.001% of the earth's crust and is chiefly mined in. Find out how tin is obtained, used and its chemical behavior. Tin is primarily obtained from the mineral cassiterite (sno 2) and is extracted by roasting cassiterite in a. Tin Atomic Mass.
From www.nuclear-power.com
Tin Atomic Number Atomic Mass Density of Tin Tin Atomic Mass Cosmically, tin is a product of neutron absorption. It has many uses, such as coating other metals, making. Tin makes up only about 0.001% of the earth's crust and is chiefly mined in. It has four valence electrons, three. Learn about its physical and chemical properties,. The atomic mass is the mass of an atom. Atomic mass of tin is. Tin Atomic Mass.
From ar.inspiredpencil.com
Tin Periodic Table Tin Atomic Mass Atomic mass of tin is 118.71 u. The atomic mass or relative isotopic mass refers to the mass. It has four valence electrons, three. Its richness in stable isotopes is noteworthy. Tin makes up only about 0.001% of the earth's crust and is chiefly mined in. Tin is primarily obtained from the mineral cassiterite (sno 2) and is extracted by. Tin Atomic Mass.
From www.dreamstime.com
Tin Chemical Element. Chemical Symbol with Atomic Number and Atomic Mass Stock Vector Tin Atomic Mass Its richness in stable isotopes is noteworthy. Find out how tin is obtained, used and its chemical behavior. Tin is primarily obtained from the mineral cassiterite (sno 2) and is extracted by roasting cassiterite in a furnace with carbon. Tin makes up only about 0.001% of the earth's crust and is chiefly mined in. Learn about its physical and chemical. Tin Atomic Mass.
From www.chemistrylearner.com
Tin Definition, Facts, Symbol, Discovery, Property, Uses Tin Atomic Mass Its richness in stable isotopes is noteworthy. Find out how tin is obtained, used and its chemical behavior. In the cosmos there are 1.33 atoms of tin per 1 × 10 6 atoms of silicon, an abundance roughly equal to that of niobium, ruthenium, neodymium, or platinum. Tin is primarily obtained from the mineral cassiterite (sno 2) and is extracted. Tin Atomic Mass.
From www.alamy.com
Sn Tin Chemical Element Periodic Table. Single vector illustration, colorful Icon with molar Tin Atomic Mass Tin makes up only about 0.001% of the earth's crust and is chiefly mined in. Its richness in stable isotopes is noteworthy. Find out how tin is obtained, used and its chemical behavior. It has many uses, such as coating other metals, making. Tin is primarily obtained from the mineral cassiterite (sno 2) and is extracted by roasting cassiterite in. Tin Atomic Mass.
From www.alamy.com
Tin (Sn). Diagram of the nuclear composition and electron configuration of an atom of tin120 Tin Atomic Mass It has many uses, such as coating other metals, making. Cosmically, tin is a product of neutron absorption. Tin is a soft, pliable metal with atomic number 50 and relative atomic mass 118.710. Its richness in stable isotopes is noteworthy. Tin makes up only about 0.001% of the earth's crust and is chiefly mined in. Tin is primarily obtained from. Tin Atomic Mass.
From www.vectorstock.com
Symbol and electron diagram for tin Royalty Free Vector Tin Atomic Mass The atomic mass is the mass of an atom. It has many uses, such as coating other metals, making. It has four valence electrons, three. The atomic mass or relative isotopic mass refers to the mass. Atomic mass of tin is 118.71 u. Tin is primarily obtained from the mineral cassiterite (sno 2) and is extracted by roasting cassiterite in. Tin Atomic Mass.
From es.slideshare.net
The Element (Tin) Tin Atomic Mass It has many uses, such as coating other metals, making. Learn about its physical and chemical properties,. Find out how tin is obtained, used and its chemical behavior. Tin is primarily obtained from the mineral cassiterite (sno 2) and is extracted by roasting cassiterite in a furnace with carbon. Tin makes up only about 0.001% of the earth's crust and. Tin Atomic Mass.
From www.dreamstime.com
Periodic Table of Elements Tin Stock Illustration Illustration of tenn, table 155333124 Tin Atomic Mass Cosmically, tin is a product of neutron absorption. Tin is primarily obtained from the mineral cassiterite (sno 2) and is extracted by roasting cassiterite in a furnace with carbon. Find out how tin is obtained, used and its chemical behavior. It has four valence electrons, three. Learn about its physical and chemical properties,. Atomic mass of tin is 118.71 u.. Tin Atomic Mass.
From www.shutterstock.com
Tin Sn Periodic Table Elements Atomic Stock Vector (Royalty Free) 2034572855 Shutterstock Tin Atomic Mass In the cosmos there are 1.33 atoms of tin per 1 × 10 6 atoms of silicon, an abundance roughly equal to that of niobium, ruthenium, neodymium, or platinum. It has many uses, such as coating other metals, making. Cosmically, tin is a product of neutron absorption. The atomic mass or relative isotopic mass refers to the mass. It has. Tin Atomic Mass.
From www.alamy.com
Tin chemical element. Chemical symbol with atomic number and atomic mass Stock Vector Image Tin Atomic Mass Tin is a silvery malleable metallic element with atomic number 50 and atomic weight 118.71. It has four valence electrons, three. The atomic mass or relative isotopic mass refers to the mass. Tin makes up only about 0.001% of the earth's crust and is chiefly mined in. Find out how tin is obtained, used and its chemical behavior. Atomic mass. Tin Atomic Mass.
From material-properties.org
Tin Periodic Table and Atomic Properties Tin Atomic Mass Tin is a silvery malleable metallic element with atomic number 50 and atomic weight 118.71. Tin is primarily obtained from the mineral cassiterite (sno 2) and is extracted by roasting cassiterite in a furnace with carbon. Atomic mass of tin is 118.71 u. Tin makes up only about 0.001% of the earth's crust and is chiefly mined in. It has. Tin Atomic Mass.
From www.storyblocks.com
Tin Atom With Element's Symbol Number Mass Stock Motion Graphics SBV300324854 Storyblocks Tin Atomic Mass It has many uses, such as coating other metals, making. Learn about its physical and chemical properties,. Tin makes up only about 0.001% of the earth's crust and is chiefly mined in. The atomic mass or relative isotopic mass refers to the mass. Tin is a soft, pliable metal with atomic number 50 and relative atomic mass 118.710. Tin is. Tin Atomic Mass.
From www.sciencephoto.com
Tin, atomic structure Stock Image C018/3731 Science Photo Library Tin Atomic Mass Find out how tin is obtained, used and its chemical behavior. The atomic mass or relative isotopic mass refers to the mass. It has four valence electrons, three. Atomic mass of tin is 118.71 u. Tin is a soft, pliable metal with atomic number 50 and relative atomic mass 118.710. Its richness in stable isotopes is noteworthy. The atomic mass. Tin Atomic Mass.
From www.dreamstime.com
Periodic Table Symbol of Tin Stock Vector Illustration of atomic, gallium 178278345 Tin Atomic Mass Tin is primarily obtained from the mineral cassiterite (sno 2) and is extracted by roasting cassiterite in a furnace with carbon. Tin is a soft, pliable metal with atomic number 50 and relative atomic mass 118.710. The atomic mass or relative isotopic mass refers to the mass. Cosmically, tin is a product of neutron absorption. Its richness in stable isotopes. Tin Atomic Mass.
From www.alamy.com
Sn Tin Chemical Element Periodic Table. Single vector illustration, element icon with molar mass Tin Atomic Mass The atomic mass or relative isotopic mass refers to the mass. Tin is primarily obtained from the mineral cassiterite (sno 2) and is extracted by roasting cassiterite in a furnace with carbon. Its richness in stable isotopes is noteworthy. Tin is a soft, pliable metal with atomic number 50 and relative atomic mass 118.710. The atomic mass is the mass. Tin Atomic Mass.
From www.alamy.com
Tin chemical element with first ionization energy, atomic mass and electronegativity on Tin Atomic Mass Tin is a silvery malleable metallic element with atomic number 50 and atomic weight 118.71. The atomic mass is the mass of an atom. Tin is a soft, pliable metal with atomic number 50 and relative atomic mass 118.710. In the cosmos there are 1.33 atoms of tin per 1 × 10 6 atoms of silicon, an abundance roughly equal. Tin Atomic Mass.
From www.istockphoto.com
Tin Chemical Element Chemical Symbol With Atomic Number And Atomic Mass Stock Illustration Tin Atomic Mass Cosmically, tin is a product of neutron absorption. The atomic mass or relative isotopic mass refers to the mass. The atomic mass is the mass of an atom. Atomic mass of tin is 118.71 u. Tin is a soft, pliable metal with atomic number 50 and relative atomic mass 118.710. Find out how tin is obtained, used and its chemical. Tin Atomic Mass.
From www.sciencephoto.com
Tin, atomic structure Stock Image C023/2540 Science Photo Library Tin Atomic Mass Tin makes up only about 0.001% of the earth's crust and is chiefly mined in. Atomic mass of tin is 118.71 u. Tin is a soft, pliable metal with atomic number 50 and relative atomic mass 118.710. The atomic mass or relative isotopic mass refers to the mass. Find out how tin is obtained, used and its chemical behavior. The. Tin Atomic Mass.
From ar.inspiredpencil.com
Tin Atomic Structure Tin Atomic Mass Find out how tin is obtained, used and its chemical behavior. Tin is primarily obtained from the mineral cassiterite (sno 2) and is extracted by roasting cassiterite in a furnace with carbon. In the cosmos there are 1.33 atoms of tin per 1 × 10 6 atoms of silicon, an abundance roughly equal to that of niobium, ruthenium, neodymium, or. Tin Atomic Mass.
From www.shutterstock.com
Tin Atomic Structure Has Atomic Number Stock Vector (Royalty Free) 1915837378 Shutterstock Tin Atomic Mass Learn about its physical and chemical properties,. Cosmically, tin is a product of neutron absorption. Atomic mass of tin is 118.71 u. The atomic mass or relative isotopic mass refers to the mass. Its richness in stable isotopes is noteworthy. It has many uses, such as coating other metals, making. Tin makes up only about 0.001% of the earth's crust. Tin Atomic Mass.
From www.nuclear-power.com
What is Tin Properties of Tin Element Symbol Sn Tin Atomic Mass It has four valence electrons, three. It has many uses, such as coating other metals, making. Tin is primarily obtained from the mineral cassiterite (sno 2) and is extracted by roasting cassiterite in a furnace with carbon. Atomic mass of tin is 118.71 u. Tin is a soft, pliable metal with atomic number 50 and relative atomic mass 118.710. Tin. Tin Atomic Mass.
From www.alamy.com
3d render of atom structure of tin isolated over white background Protons are represented as red Tin Atomic Mass Its richness in stable isotopes is noteworthy. Tin is a soft, pliable metal with atomic number 50 and relative atomic mass 118.710. Tin is primarily obtained from the mineral cassiterite (sno 2) and is extracted by roasting cassiterite in a furnace with carbon. It has four valence electrons, three. Learn about its physical and chemical properties,. The atomic mass is. Tin Atomic Mass.
From www.alamy.com
Tin chemical element. Chemical symbol with atomic number and atomic mass. illustration Stock Tin Atomic Mass The atomic mass or relative isotopic mass refers to the mass. It has many uses, such as coating other metals, making. It has four valence electrons, three. The atomic mass is the mass of an atom. Its richness in stable isotopes is noteworthy. Atomic mass of tin is 118.71 u. Find out how tin is obtained, used and its chemical. Tin Atomic Mass.
From pngtree.com
Chemical Properties Of Tin Atomic Symbol With Number And Mass Vector, Neutron, Object, Science Tin Atomic Mass Learn about its physical and chemical properties,. It has many uses, such as coating other metals, making. Atomic mass of tin is 118.71 u. In the cosmos there are 1.33 atoms of tin per 1 × 10 6 atoms of silicon, an abundance roughly equal to that of niobium, ruthenium, neodymium, or platinum. Tin is a soft, pliable metal with. Tin Atomic Mass.
From www.britannica.com
Tin Definition, Properties, Uses, & Facts Britannica Tin Atomic Mass Tin is a silvery malleable metallic element with atomic number 50 and atomic weight 118.71. It has many uses, such as coating other metals, making. Atomic mass of tin is 118.71 u. Its richness in stable isotopes is noteworthy. Tin makes up only about 0.001% of the earth's crust and is chiefly mined in. It has four valence electrons, three.. Tin Atomic Mass.
From www.chemistrylearner.com
Tin Definition, Facts, Symbol, Discovery, Property, Uses Tin Atomic Mass The atomic mass is the mass of an atom. In the cosmos there are 1.33 atoms of tin per 1 × 10 6 atoms of silicon, an abundance roughly equal to that of niobium, ruthenium, neodymium, or platinum. It has many uses, such as coating other metals, making. Tin is primarily obtained from the mineral cassiterite (sno 2) and is. Tin Atomic Mass.
From www.dreamstime.com
Round Periodic Table Element Symbol of Tin Stock Vector Illustration of vector, argon 178670616 Tin Atomic Mass Tin is a silvery malleable metallic element with atomic number 50 and atomic weight 118.71. Cosmically, tin is a product of neutron absorption. Tin makes up only about 0.001% of the earth's crust and is chiefly mined in. In the cosmos there are 1.33 atoms of tin per 1 × 10 6 atoms of silicon, an abundance roughly equal to. Tin Atomic Mass.
From www.alamy.com
Tin chemical element. Chemical symbol with atomic number and atomic mass. illustration Stock Tin Atomic Mass The atomic mass or relative isotopic mass refers to the mass. Learn about its physical and chemical properties,. It has many uses, such as coating other metals, making. Cosmically, tin is a product of neutron absorption. The atomic mass is the mass of an atom. Tin is a soft, pliable metal with atomic number 50 and relative atomic mass 118.710.. Tin Atomic Mass.
From periodictableguide.com
Tin (Sn) Periodic Table (Element Information & More) Tin Atomic Mass Tin makes up only about 0.001% of the earth's crust and is chiefly mined in. Tin is a soft, pliable metal with atomic number 50 and relative atomic mass 118.710. Atomic mass of tin is 118.71 u. It has four valence electrons, three. Find out how tin is obtained, used and its chemical behavior. Its richness in stable isotopes is. Tin Atomic Mass.
From www.sciencephoto.com
Tin, atomic structure Stock Image C045/6393 Science Photo Library Tin Atomic Mass The atomic mass or relative isotopic mass refers to the mass. Tin is primarily obtained from the mineral cassiterite (sno 2) and is extracted by roasting cassiterite in a furnace with carbon. Cosmically, tin is a product of neutron absorption. It has four valence electrons, three. The atomic mass is the mass of an atom. Its richness in stable isotopes. Tin Atomic Mass.
From www.dreamstime.com
Tin Atom, with Mass and Energy Levels. Stock Vector Illustration of formula, periodic 256806989 Tin Atomic Mass Learn about its physical and chemical properties,. Tin is a soft, pliable metal with atomic number 50 and relative atomic mass 118.710. Find out how tin is obtained, used and its chemical behavior. The atomic mass or relative isotopic mass refers to the mass. Tin makes up only about 0.001% of the earth's crust and is chiefly mined in. It. Tin Atomic Mass.
From ar.inspiredpencil.com
Tin Atomic Structure Tin Atomic Mass Tin is primarily obtained from the mineral cassiterite (sno 2) and is extracted by roasting cassiterite in a furnace with carbon. Tin is a soft, pliable metal with atomic number 50 and relative atomic mass 118.710. Tin makes up only about 0.001% of the earth's crust and is chiefly mined in. Cosmically, tin is a product of neutron absorption. The. Tin Atomic Mass.