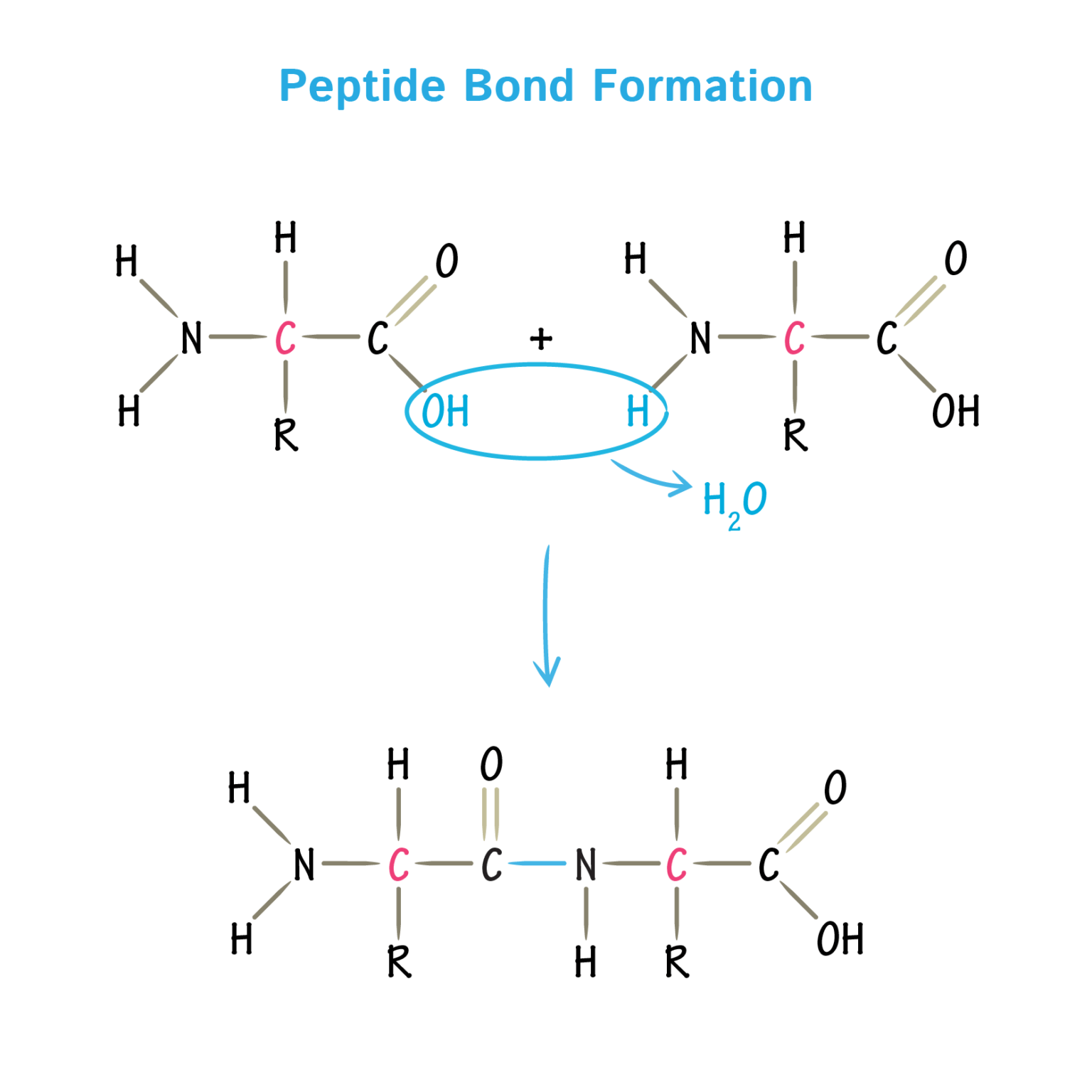
Amino Acid Carboxyl Group Condensation Reaction . A peptide is a combination of amino acids in which the amine group of one amino acid has undergone a reaction with the carboxyl group of another. In this reaction the carboxylic acid adds to the dcc molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. It is well known that peptides are synthesized by a so called condensation reaction between an amine and a carboxylic acid group to form the final amide moiety (because it. The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used. A condensation reaction between the carboxyl group of alanine and and the amino group of valine generates a peptide bond linking the two amino acids, with a molecule.
from microbiologynotes.org
The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used. A peptide is a combination of amino acids in which the amine group of one amino acid has undergone a reaction with the carboxyl group of another. It is well known that peptides are synthesized by a so called condensation reaction between an amine and a carboxylic acid group to form the final amide moiety (because it. A condensation reaction between the carboxyl group of alanine and and the amino group of valine generates a peptide bond linking the two amino acids, with a molecule. In this reaction the carboxylic acid adds to the dcc molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution.
Amino acids physical, chemical properties and peptide bond
Amino Acid Carboxyl Group Condensation Reaction It is well known that peptides are synthesized by a so called condensation reaction between an amine and a carboxylic acid group to form the final amide moiety (because it. The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used. In this reaction the carboxylic acid adds to the dcc molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. It is well known that peptides are synthesized by a so called condensation reaction between an amine and a carboxylic acid group to form the final amide moiety (because it. A condensation reaction between the carboxyl group of alanine and and the amino group of valine generates a peptide bond linking the two amino acids, with a molecule. A peptide is a combination of amino acids in which the amine group of one amino acid has undergone a reaction with the carboxyl group of another.
From www.researchgate.net
4. Two amino acids (the carboxylicgroup from the amino acid 1 and the Amino Acid Carboxyl Group Condensation Reaction In this reaction the carboxylic acid adds to the dcc molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. A peptide is a combination of amino acids in which the amine group of one amino acid has undergone a reaction with the carboxyl group of another. A condensation reaction between the. Amino Acid Carboxyl Group Condensation Reaction.
From www.animalia-life.club
Amino Group And Carboxyl Group Amino Acid Carboxyl Group Condensation Reaction A condensation reaction between the carboxyl group of alanine and and the amino group of valine generates a peptide bond linking the two amino acids, with a molecule. In this reaction the carboxylic acid adds to the dcc molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. A peptide is a. Amino Acid Carboxyl Group Condensation Reaction.
From www.researchgate.net
Condensation reaction on mineral surfaces, where activated monomers Amino Acid Carboxyl Group Condensation Reaction A condensation reaction between the carboxyl group of alanine and and the amino group of valine generates a peptide bond linking the two amino acids, with a molecule. In this reaction the carboxylic acid adds to the dcc molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. A peptide is a. Amino Acid Carboxyl Group Condensation Reaction.
From microbenotes.com
Amino Acids Properties, Structure, Classification, Functions Amino Acid Carboxyl Group Condensation Reaction It is well known that peptides are synthesized by a so called condensation reaction between an amine and a carboxylic acid group to form the final amide moiety (because it. A peptide is a combination of amino acids in which the amine group of one amino acid has undergone a reaction with the carboxyl group of another. A condensation reaction. Amino Acid Carboxyl Group Condensation Reaction.
From www.masterorganicchemistry.com
Protecting Groups for Amines Carbamates Master Organic Chemistry Amino Acid Carboxyl Group Condensation Reaction The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used. A condensation reaction between the carboxyl group of alanine and and the amino group of valine generates a peptide bond linking the two amino acids, with a molecule. It is well known that peptides are synthesized by a so. Amino Acid Carboxyl Group Condensation Reaction.
From www.masterorganicchemistry.com
Conversion of carboxylic acids to esters using acid and alcohols Amino Acid Carboxyl Group Condensation Reaction A condensation reaction between the carboxyl group of alanine and and the amino group of valine generates a peptide bond linking the two amino acids, with a molecule. The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used. In this reaction the carboxylic acid adds to the dcc molecule. Amino Acid Carboxyl Group Condensation Reaction.
From www.slideserve.com
PPT Amine Reactions PowerPoint Presentation, free download ID2118635 Amino Acid Carboxyl Group Condensation Reaction In this reaction the carboxylic acid adds to the dcc molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used. It is well known that peptides are synthesized by a so. Amino Acid Carboxyl Group Condensation Reaction.
From www.slideserve.com
PPT Lecture 2 Eukaryotic cells, amino acids PowerPoint Presentation Amino Acid Carboxyl Group Condensation Reaction A condensation reaction between the carboxyl group of alanine and and the amino group of valine generates a peptide bond linking the two amino acids, with a molecule. A peptide is a combination of amino acids in which the amine group of one amino acid has undergone a reaction with the carboxyl group of another. In this reaction the carboxylic. Amino Acid Carboxyl Group Condensation Reaction.
From www.herongyang.com
Peptide, Peptide Bond, Amino Acid Residues Amino Acid Carboxyl Group Condensation Reaction A peptide is a combination of amino acids in which the amine group of one amino acid has undergone a reaction with the carboxyl group of another. The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used. A condensation reaction between the carboxyl group of alanine and and the. Amino Acid Carboxyl Group Condensation Reaction.
From www.numerade.com
SOLVED Amino Acids Amino acids may undergo condensation reactions to Amino Acid Carboxyl Group Condensation Reaction The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used. In this reaction the carboxylic acid adds to the dcc molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. It is well known that peptides are synthesized by a so. Amino Acid Carboxyl Group Condensation Reaction.
From www.slideserve.com
PPT Carboxylic Acids Reactions PowerPoint Presentation, free Amino Acid Carboxyl Group Condensation Reaction The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used. In this reaction the carboxylic acid adds to the dcc molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. A peptide is a combination of amino acids in which the. Amino Acid Carboxyl Group Condensation Reaction.
From www.expii.com
Amino Acids — Overview & Structure Expii Amino Acid Carboxyl Group Condensation Reaction The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used. In this reaction the carboxylic acid adds to the dcc molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. A peptide is a combination of amino acids in which the. Amino Acid Carboxyl Group Condensation Reaction.
From www.chemistrysteps.com
Amides from Carboxylic AcidsDCC and EDC Coupling Chemistry Steps Amino Acid Carboxyl Group Condensation Reaction The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used. It is well known that peptides are synthesized by a so called condensation reaction between an amine and a carboxylic acid group to form the final amide moiety (because it. A condensation reaction between the carboxyl group of alanine. Amino Acid Carboxyl Group Condensation Reaction.
From d15.beauty
Condensation Reaction Mechanism Amino Acid Carboxyl Group Condensation Reaction In this reaction the carboxylic acid adds to the dcc molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. A condensation reaction between the carboxyl group of alanine and and the amino group of valine generates a peptide bond linking the two amino acids, with a molecule. A peptide is a. Amino Acid Carboxyl Group Condensation Reaction.
From ar.inspiredpencil.com
Condensation Reaction Between Alcohol And Carboxylic Acid Amino Acid Carboxyl Group Condensation Reaction It is well known that peptides are synthesized by a so called condensation reaction between an amine and a carboxylic acid group to form the final amide moiety (because it. In this reaction the carboxylic acid adds to the dcc molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. The coupling. Amino Acid Carboxyl Group Condensation Reaction.
From www.researchgate.net
Condensation mechanism of bamino acid between carboxylates and other Amino Acid Carboxyl Group Condensation Reaction The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used. It is well known that peptides are synthesized by a so called condensation reaction between an amine and a carboxylic acid group to form the final amide moiety (because it. A condensation reaction between the carboxyl group of alanine. Amino Acid Carboxyl Group Condensation Reaction.
From slidetodoc.com
Chapter 3 Notes Biochemistry the chemistry of life Amino Acid Carboxyl Group Condensation Reaction It is well known that peptides are synthesized by a so called condensation reaction between an amine and a carboxylic acid group to form the final amide moiety (because it. A peptide is a combination of amino acids in which the amine group of one amino acid has undergone a reaction with the carboxyl group of another. A condensation reaction. Amino Acid Carboxyl Group Condensation Reaction.
From www.researchgate.net
Reaction of carboxylic acid and amine to form an amide Download Amino Acid Carboxyl Group Condensation Reaction In this reaction the carboxylic acid adds to the dcc molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. It is well known that peptides are synthesized by a so called condensation reaction between an amine and a carboxylic acid group to form the final amide moiety (because it. A peptide. Amino Acid Carboxyl Group Condensation Reaction.
From ar.inspiredpencil.com
The Reaction Between Two Amino Acids Amino Acid Carboxyl Group Condensation Reaction It is well known that peptides are synthesized by a so called condensation reaction between an amine and a carboxylic acid group to form the final amide moiety (because it. In this reaction the carboxylic acid adds to the dcc molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. The coupling. Amino Acid Carboxyl Group Condensation Reaction.
From mavink.com
Amino Acid Structure Labeled Amino Acid Carboxyl Group Condensation Reaction In this reaction the carboxylic acid adds to the dcc molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. It is well known that peptides are synthesized by a so called condensation reaction between an amine and a carboxylic acid group to form the final amide moiety (because it. A condensation. Amino Acid Carboxyl Group Condensation Reaction.
From ar.inspiredpencil.com
Condensation Reaction Between Alcohol And Carboxylic Acid Amino Acid Carboxyl Group Condensation Reaction The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used. A condensation reaction between the carboxyl group of alanine and and the amino group of valine generates a peptide bond linking the two amino acids, with a molecule. A peptide is a combination of amino acids in which the. Amino Acid Carboxyl Group Condensation Reaction.
From philschatz.com
Amines and Amides · Chemistry Amino Acid Carboxyl Group Condensation Reaction The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used. A condensation reaction between the carboxyl group of alanine and and the amino group of valine generates a peptide bond linking the two amino acids, with a molecule. It is well known that peptides are synthesized by a so. Amino Acid Carboxyl Group Condensation Reaction.
From ar.inspiredpencil.com
Amino Acid Condensation Reaction Mechanism Amino Acid Carboxyl Group Condensation Reaction The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used. In this reaction the carboxylic acid adds to the dcc molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. A condensation reaction between the carboxyl group of alanine and and. Amino Acid Carboxyl Group Condensation Reaction.
From jackwestin.com
Important Reactions Acid Derivatives MCAT Content Amino Acid Carboxyl Group Condensation Reaction A condensation reaction between the carboxyl group of alanine and and the amino group of valine generates a peptide bond linking the two amino acids, with a molecule. It is well known that peptides are synthesized by a so called condensation reaction between an amine and a carboxylic acid group to form the final amide moiety (because it. A peptide. Amino Acid Carboxyl Group Condensation Reaction.
From www.britannica.com
Amino acid Reactions, Structure, Synthesis Britannica Amino Acid Carboxyl Group Condensation Reaction It is well known that peptides are synthesized by a so called condensation reaction between an amine and a carboxylic acid group to form the final amide moiety (because it. The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used. A peptide is a combination of amino acids in. Amino Acid Carboxyl Group Condensation Reaction.
From www.dreamstime.com
Peptide Bond. Formation of Amide Bonds from Two Amino Acids As a Result Amino Acid Carboxyl Group Condensation Reaction In this reaction the carboxylic acid adds to the dcc molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used. A condensation reaction between the carboxyl group of alanine and and. Amino Acid Carboxyl Group Condensation Reaction.
From www.youtube.com
Condensation Reaction of Amine + Carboxylic Acid YouTube Amino Acid Carboxyl Group Condensation Reaction The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used. A peptide is a combination of amino acids in which the amine group of one amino acid has undergone a reaction with the carboxyl group of another. It is well known that peptides are synthesized by a so called. Amino Acid Carboxyl Group Condensation Reaction.
From ar.inspiredpencil.com
Amino Acid Condensation Reaction Mechanism Amino Acid Carboxyl Group Condensation Reaction The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used. In this reaction the carboxylic acid adds to the dcc molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. A condensation reaction between the carboxyl group of alanine and and. Amino Acid Carboxyl Group Condensation Reaction.
From slideplayer.com
Proteins Basic structure of an amino acid ppt download Amino Acid Carboxyl Group Condensation Reaction A condensation reaction between the carboxyl group of alanine and and the amino group of valine generates a peptide bond linking the two amino acids, with a molecule. It is well known that peptides are synthesized by a so called condensation reaction between an amine and a carboxylic acid group to form the final amide moiety (because it. A peptide. Amino Acid Carboxyl Group Condensation Reaction.
From ar.inspiredpencil.com
Amino Acid Condensation Reaction Mechanism Amino Acid Carboxyl Group Condensation Reaction A condensation reaction between the carboxyl group of alanine and and the amino group of valine generates a peptide bond linking the two amino acids, with a molecule. It is well known that peptides are synthesized by a so called condensation reaction between an amine and a carboxylic acid group to form the final amide moiety (because it. In this. Amino Acid Carboxyl Group Condensation Reaction.
From www.researchgate.net
Schematic reaction of the condensation of carboxyl groups of Amino Acid Carboxyl Group Condensation Reaction It is well known that peptides are synthesized by a so called condensation reaction between an amine and a carboxylic acid group to form the final amide moiety (because it. In this reaction the carboxylic acid adds to the dcc molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. A condensation. Amino Acid Carboxyl Group Condensation Reaction.
From slideplayer.com
Carboxylic Acids Reactions ppt download Amino Acid Carboxyl Group Condensation Reaction A peptide is a combination of amino acids in which the amine group of one amino acid has undergone a reaction with the carboxyl group of another. The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used. A condensation reaction between the carboxyl group of alanine and and the. Amino Acid Carboxyl Group Condensation Reaction.
From www.researchgate.net
A peptide bond is formed between two amino acid molecules when the Amino Acid Carboxyl Group Condensation Reaction In this reaction the carboxylic acid adds to the dcc molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. A condensation reaction between the carboxyl group of alanine and and the amino group of valine generates a peptide bond linking the two amino acids, with a molecule. A peptide is a. Amino Acid Carboxyl Group Condensation Reaction.
From microbiologynotes.org
Amino acids physical, chemical properties and peptide bond Amino Acid Carboxyl Group Condensation Reaction A condensation reaction between the carboxyl group of alanine and and the amino group of valine generates a peptide bond linking the two amino acids, with a molecule. It is well known that peptides are synthesized by a so called condensation reaction between an amine and a carboxylic acid group to form the final amide moiety (because it. In this. Amino Acid Carboxyl Group Condensation Reaction.
From hubpages.com
What Are Proteins? Making and Breaking Proteins HubPages Amino Acid Carboxyl Group Condensation Reaction In this reaction the carboxylic acid adds to the dcc molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. It is well known that peptides are synthesized by a so called condensation reaction between an amine and a carboxylic acid group to form the final amide moiety (because it. A condensation. Amino Acid Carboxyl Group Condensation Reaction.