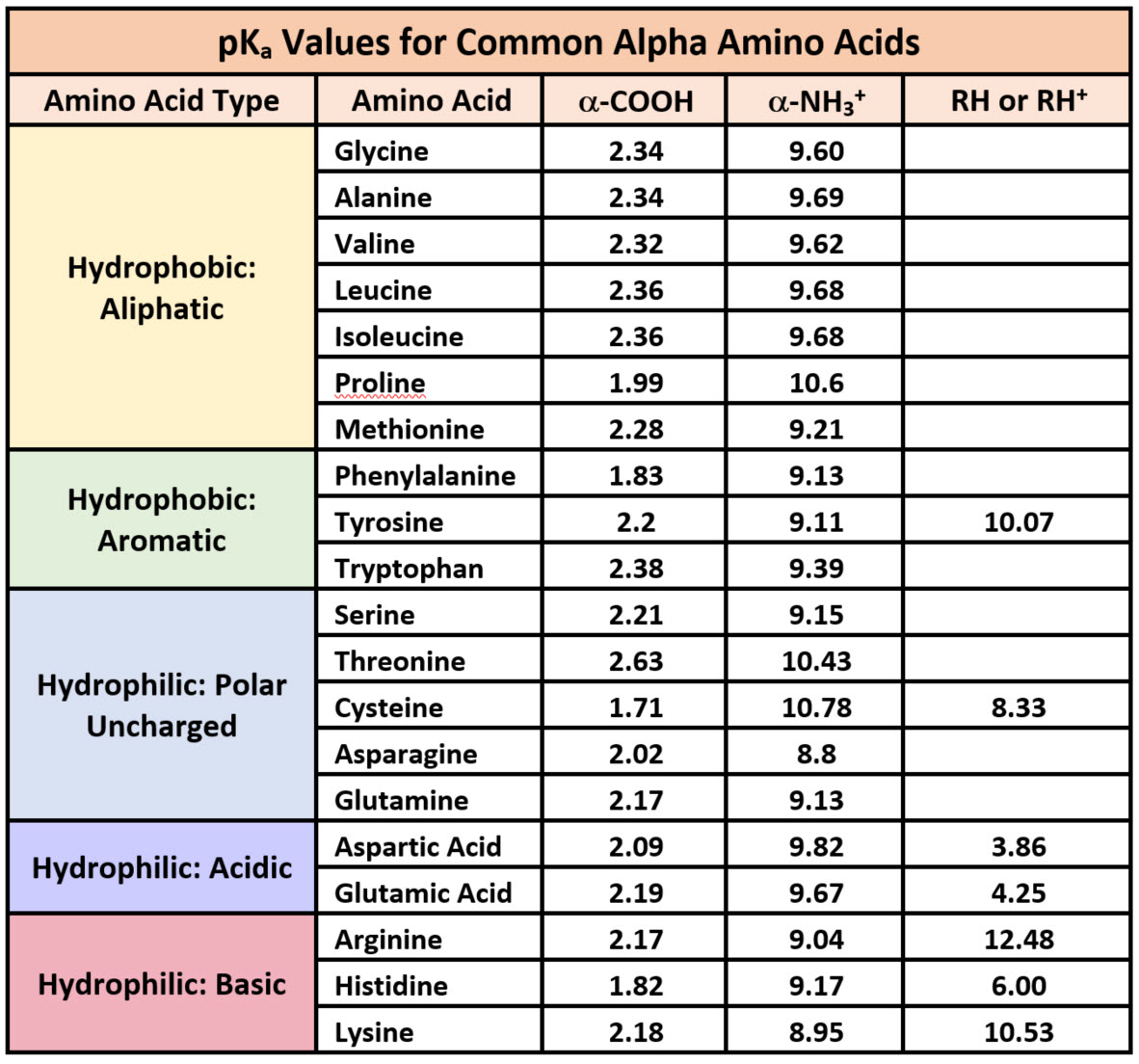
Arginine Charge At Ph 8 . The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. You are right in your reasoning: Determine the net charge of histidine at ph 1, 5, 9.17, and 12. I have been given $\mathrm pk_\mathrm a$ values of an amino group, a carboxyl group and a side chain of cysteine. There are three amino acids that have basic side chains at neutral ph. As a result, the only remaining charge will be on the carboxylate ion so the amino acid will have a. These are arginine (arg), lysine (lys), and histidine (his). At any ph for any titratable group, there is a distribution between the protonated and deprotonated species. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. We can calculate this distribution and therefore the. How can i find the ionic charge on it at different. The solute molecules of arginine therefore carry an excess positive charge, and. Both base functions exist as onium conjugate acids in the ph 6.00 matrix. The two carboxyl functions in. Arginine is a basic amino acid.
from cameronlawrence.z21.web.core.windows.net
I have been given $\mathrm pk_\mathrm a$ values of an amino group, a carboxyl group and a side chain of cysteine. You are right in your reasoning: The solute molecules of arginine therefore carry an excess positive charge, and. The two carboxyl functions in. There are three amino acids that have basic side chains at neutral ph. We can calculate this distribution and therefore the. These are arginine (arg), lysine (lys), and histidine (his). The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. Arginine is a basic amino acid. Both base functions exist as onium conjugate acids in the ph 6.00 matrix.
Pka Chart Organic Chemistry
Arginine Charge At Ph 8 How can i find the ionic charge on it at different. We can calculate this distribution and therefore the. You are right in your reasoning: At any ph for any titratable group, there is a distribution between the protonated and deprotonated species. As a result, the only remaining charge will be on the carboxylate ion so the amino acid will have a. The solute molecules of arginine therefore carry an excess positive charge, and. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. Both base functions exist as onium conjugate acids in the ph 6.00 matrix. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. I have been given $\mathrm pk_\mathrm a$ values of an amino group, a carboxyl group and a side chain of cysteine. There are three amino acids that have basic side chains at neutral ph. Arginine is a basic amino acid. These are arginine (arg), lysine (lys), and histidine (his). How can i find the ionic charge on it at different. The two carboxyl functions in. Determine the net charge of histidine at ph 1, 5, 9.17, and 12.
From www.copmed.fr
LArginine Arginine Charge At Ph 8 The two carboxyl functions in. Determine the net charge of histidine at ph 1, 5, 9.17, and 12. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. The solute molecules of arginine therefore carry an excess positive charge, and. Both base functions exist as onium conjugate acids in the ph 6.00 matrix.. Arginine Charge At Ph 8.
From www.numerade.com
SOLVED At physiological pH, the side chain of the amino acid arginine Arginine Charge At Ph 8 The two carboxyl functions in. Both base functions exist as onium conjugate acids in the ph 6.00 matrix. Arginine is a basic amino acid. As a result, the only remaining charge will be on the carboxylate ion so the amino acid will have a. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the. Arginine Charge At Ph 8.
From thepeacechallenge.blogspot.com
Arginine Amino Acid Charge Brain Mind Article Arginine Charge At Ph 8 You are right in your reasoning: These are arginine (arg), lysine (lys), and histidine (his). How can i find the ionic charge on it at different. Determine the net charge of histidine at ph 1, 5, 9.17, and 12. There are three amino acids that have basic side chains at neutral ph. Both base functions exist as onium conjugate acids. Arginine Charge At Ph 8.
From www.dreamstime.com
Arginine Chemical Structure. Vector Illustration Hand Drawn Stock Arginine Charge At Ph 8 Arginine is a basic amino acid. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. How can i find the ionic charge on it at different. As a result, the only remaining charge will be on the carboxylate ion so the amino acid will have a. These are arginine (arg), lysine (lys),. Arginine Charge At Ph 8.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master Arginine Charge At Ph 8 As a result, the only remaining charge will be on the carboxylate ion so the amino acid will have a. I have been given $\mathrm pk_\mathrm a$ values of an amino group, a carboxyl group and a side chain of cysteine. You are right in your reasoning: At any ph for any titratable group, there is a distribution between the. Arginine Charge At Ph 8.
From www.researchgate.net
Amino Acid pKa and corresponding protonation states at low, medium, and Arginine Charge At Ph 8 If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. Arginine is a basic amino acid. The solute molecules of arginine therefore carry an excess positive charge, and. You are right in your reasoning: These are arginine (arg), lysine (lys), and histidine (his). Both base functions exist as onium conjugate. Arginine Charge At Ph 8.
From www.chegg.com
Solved 8. The amino acid arginine (shown to the right) has Arginine Charge At Ph 8 The two carboxyl functions in. You are right in your reasoning: I have been given $\mathrm pk_\mathrm a$ values of an amino group, a carboxyl group and a side chain of cysteine. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. As a result, the only remaining charge will. Arginine Charge At Ph 8.
From www.slideserve.com
PPT Amino Acids and the Primary Structures of Proteins PowerPoint Arginine Charge At Ph 8 How can i find the ionic charge on it at different. Determine the net charge of histidine at ph 1, 5, 9.17, and 12. You are right in your reasoning: If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. There are three amino acids that have basic side chains. Arginine Charge At Ph 8.
From www.alamy.it
Formula chimica dell'arginina. Struttura molecolare chimica dell Arginine Charge At Ph 8 As a result, the only remaining charge will be on the carboxylate ion so the amino acid will have a. Determine the net charge of histidine at ph 1, 5, 9.17, and 12. How can i find the ionic charge on it at different. I have been given $\mathrm pk_\mathrm a$ values of an amino group, a carboxyl group and. Arginine Charge At Ph 8.
From thepeacechallenge.blogspot.com
Arginine Positive Charge Brain Mind Article Arginine Charge At Ph 8 If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. These are arginine (arg), lysine (lys), and histidine (his). I have been given $\mathrm pk_\mathrm a$ values of an amino group, a carboxyl group and a side chain of cysteine. Arginine is a basic amino acid. The solute molecules of. Arginine Charge At Ph 8.
From www.chegg.com
Solved Draw the structure of arginine in the protonation Arginine Charge At Ph 8 Both base functions exist as onium conjugate acids in the ph 6.00 matrix. You are right in your reasoning: Determine the net charge of histidine at ph 1, 5, 9.17, and 12. Arginine is a basic amino acid. How can i find the ionic charge on it at different. The solute molecules of arginine therefore carry an excess positive charge,. Arginine Charge At Ph 8.
From www.researchgate.net
Positively charged amino acids arginine and lysine, and hydrogen Arginine Charge At Ph 8 These are arginine (arg), lysine (lys), and histidine (his). The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. How can i find the ionic charge on it at different. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. The solute molecules. Arginine Charge At Ph 8.
From www.chegg.com
Solved 4. (10 points) Take a look at the arginine titration Arginine Charge At Ph 8 Arginine is a basic amino acid. How can i find the ionic charge on it at different. Determine the net charge of histidine at ph 1, 5, 9.17, and 12. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. I have been given $\mathrm pk_\mathrm a$ values of an. Arginine Charge At Ph 8.
From www.chegg.com
Solved Draw the dominant forms of LArginine at the Arginine Charge At Ph 8 Determine the net charge of histidine at ph 1, 5, 9.17, and 12. As a result, the only remaining charge will be on the carboxylate ion so the amino acid will have a. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. The solute molecules of arginine therefore carry. Arginine Charge At Ph 8.
From www.alamy.com
Amino acid arginine molecular structure on a white background Stock Arginine Charge At Ph 8 You are right in your reasoning: Determine the net charge of histidine at ph 1, 5, 9.17, and 12. At any ph for any titratable group, there is a distribution between the protonated and deprotonated species. Both base functions exist as onium conjugate acids in the ph 6.00 matrix. We can calculate this distribution and therefore the. Arginine is a. Arginine Charge At Ph 8.
From www.numerade.com
SOLVED Arginine has the following pKa values pK1 = 2.17, pK2 = 9.04 Arginine Charge At Ph 8 The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. The two carboxyl functions in. Arginine is a basic amino acid. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. I have been given $\mathrm pk_\mathrm a$ values of an amino group,. Arginine Charge At Ph 8.
From www.chegg.com
Solved What is the net charge of arginine at pH 7? Arginine Charge At Ph 8 At any ph for any titratable group, there is a distribution between the protonated and deprotonated species. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. Determine the net charge of histidine at ph 1, 5, 9.17, and 12. There are three amino acids that have basic side chains at neutral ph.. Arginine Charge At Ph 8.
From ar.inspiredpencil.com
Arginine Structure Arginine Charge At Ph 8 Both base functions exist as onium conjugate acids in the ph 6.00 matrix. Determine the net charge of histidine at ph 1, 5, 9.17, and 12. These are arginine (arg), lysine (lys), and histidine (his). At any ph for any titratable group, there is a distribution between the protonated and deprotonated species. How can i find the ionic charge on. Arginine Charge At Ph 8.
From foodsciencetoolbox.com
How to Determine the Net Charge of Amino Acids Food Science Toolbox Arginine Charge At Ph 8 Both base functions exist as onium conjugate acids in the ph 6.00 matrix. As a result, the only remaining charge will be on the carboxylate ion so the amino acid will have a. There are three amino acids that have basic side chains at neutral ph. At any ph for any titratable group, there is a distribution between the protonated. Arginine Charge At Ph 8.
From onlinelibrary.wiley.com
Arginine Its pKa value revisited Fitch 2015 Protein Science Arginine Charge At Ph 8 At any ph for any titratable group, there is a distribution between the protonated and deprotonated species. You are right in your reasoning: I have been given $\mathrm pk_\mathrm a$ values of an amino group, a carboxyl group and a side chain of cysteine. How can i find the ionic charge on it at different. The two carboxyl functions in.. Arginine Charge At Ph 8.
From www.chegg.com
Solved What is the net charge of arginine in a solution of Arginine Charge At Ph 8 Both base functions exist as onium conjugate acids in the ph 6.00 matrix. Arginine is a basic amino acid. These are arginine (arg), lysine (lys), and histidine (his). I have been given $\mathrm pk_\mathrm a$ values of an amino group, a carboxyl group and a side chain of cysteine. Determine the net charge of histidine at ph 1, 5, 9.17,. Arginine Charge At Ph 8.
From www.vectorstock.com
Arginine amino acid molecule skeletal formula Vector Image Arginine Charge At Ph 8 The solute molecules of arginine therefore carry an excess positive charge, and. At any ph for any titratable group, there is a distribution between the protonated and deprotonated species. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. We can calculate this distribution and therefore the. You are right. Arginine Charge At Ph 8.
From www.researchgate.net
Electrochemical structures for various Larginine analogues and organic Arginine Charge At Ph 8 How can i find the ionic charge on it at different. Both base functions exist as onium conjugate acids in the ph 6.00 matrix. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. The solute molecules of arginine therefore carry an excess positive charge, and. Arginine is a basic. Arginine Charge At Ph 8.
From thepeacechallenge.blogspot.com
Arginine Charge At Ph 8 Brain Mind Article Arginine Charge At Ph 8 Determine the net charge of histidine at ph 1, 5, 9.17, and 12. There are three amino acids that have basic side chains at neutral ph. Both base functions exist as onium conjugate acids in the ph 6.00 matrix. These are arginine (arg), lysine (lys), and histidine (his). The solute molecules of arginine therefore carry an excess positive charge, and. Arginine Charge At Ph 8.
From it.dreamstime.com
Formula Chimica Dell'arginina Illustrazione Vettoriale Illustrazione Arginine Charge At Ph 8 Determine the net charge of histidine at ph 1, 5, 9.17, and 12. There are three amino acids that have basic side chains at neutral ph. Both base functions exist as onium conjugate acids in the ph 6.00 matrix. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. We. Arginine Charge At Ph 8.
From www.chegg.com
Solved 1.(a) At pH of 12, the net charge of arginine is Arginine Charge At Ph 8 There are three amino acids that have basic side chains at neutral ph. As a result, the only remaining charge will be on the carboxylate ion so the amino acid will have a. Determine the net charge of histidine at ph 1, 5, 9.17, and 12. We can calculate this distribution and therefore the. The solute molecules of arginine therefore. Arginine Charge At Ph 8.
From www.chegg.com
Solved Part AHand draw the structures of amino acids Arginine Charge At Ph 8 Determine the net charge of histidine at ph 1, 5, 9.17, and 12. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. How can i find the ionic charge on it at different. We can calculate this distribution and therefore the. Arginine is a basic amino acid. There are three amino acids. Arginine Charge At Ph 8.
From www.chegg.com
Solved What will be the predominant form of arginine at pH Arginine Charge At Ph 8 As a result, the only remaining charge will be on the carboxylate ion so the amino acid will have a. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. The solute molecules. Arginine Charge At Ph 8.
From gbee.edu.vn
Isoelectric Points of Amino Acids (and How To Calculate Them) Gbee Arginine Charge At Ph 8 We can calculate this distribution and therefore the. How can i find the ionic charge on it at different. The solute molecules of arginine therefore carry an excess positive charge, and. There are three amino acids that have basic side chains at neutral ph. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the. Arginine Charge At Ph 8.
From www.shutterstock.com
Arginine Arg R Amino Acid Molecular Stock Vector (Royalty Free Arginine Charge At Ph 8 How can i find the ionic charge on it at different. You are right in your reasoning: The solute molecules of arginine therefore carry an excess positive charge, and. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. At any ph for any titratable group, there is a distribution. Arginine Charge At Ph 8.
From chemistryjee.blogspot.com
chemistry world Arginine Charge At Ph 8 We can calculate this distribution and therefore the. There are three amino acids that have basic side chains at neutral ph. The two carboxyl functions in. Both base functions exist as onium conjugate acids in the ph 6.00 matrix. Arginine is a basic amino acid. Determine the net charge of histidine at ph 1, 5, 9.17, and 12. The solute. Arginine Charge At Ph 8.
From cameronlawrence.z21.web.core.windows.net
Pka Chart Organic Chemistry Arginine Charge At Ph 8 We can calculate this distribution and therefore the. Arginine is a basic amino acid. Both base functions exist as onium conjugate acids in the ph 6.00 matrix. Determine the net charge of histidine at ph 1, 5, 9.17, and 12. As a result, the only remaining charge will be on the carboxylate ion so the amino acid will have a.. Arginine Charge At Ph 8.
From de.academic.ru
LArginin Arginine Charge At Ph 8 There are three amino acids that have basic side chains at neutral ph. These are arginine (arg), lysine (lys), and histidine (his). The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. Arginine is a basic amino acid. We can calculate this distribution and therefore the. How can i find the ionic charge. Arginine Charge At Ph 8.
From www.alamy.com
Arginine Amino Acid Molecule Ball and Stick Structure Stock Vector Arginine Charge At Ph 8 I have been given $\mathrm pk_\mathrm a$ values of an amino group, a carboxyl group and a side chain of cysteine. As a result, the only remaining charge will be on the carboxylate ion so the amino acid will have a. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. You are. Arginine Charge At Ph 8.
From bceweb.org
Amino Acid Ph Chart A Visual Reference of Charts Chart Master Arginine Charge At Ph 8 I have been given $\mathrm pk_\mathrm a$ values of an amino group, a carboxyl group and a side chain of cysteine. We can calculate this distribution and therefore the. The solute molecules of arginine therefore carry an excess positive charge, and. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. Determine the. Arginine Charge At Ph 8.