Copper Ii Nitrate Dissolved In Water Equation . This reaction is a cheap source of. In this chapter, we will focus on solution where the solvent is water. The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility, defined as the maximum. $$ \ce{3cu (s) + 2no3. Copper (ii) nitrate, often recognized by its chemical formula cu(no 3) 2, is an inorganic compound that has significant utility in a variety of industries and. 2 cu (no 3) 2 → 2 cuo + 4 no 2 + o 2. 133 rows solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the rate. Decomposition of copper nitrate yields nitrogen dioxide gas. An aqueous solution is water that contains one or more dissolved substance. Copper is a much better reducing agent than water:
from www.numerade.com
Copper (ii) nitrate, often recognized by its chemical formula cu(no 3) 2, is an inorganic compound that has significant utility in a variety of industries and. In this chapter, we will focus on solution where the solvent is water. 2 cu (no 3) 2 → 2 cuo + 4 no 2 + o 2. An aqueous solution is water that contains one or more dissolved substance. 133 rows solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the rate. Decomposition of copper nitrate yields nitrogen dioxide gas. Copper is a much better reducing agent than water: $$ \ce{3cu (s) + 2no3. This reaction is a cheap source of. The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility, defined as the maximum.
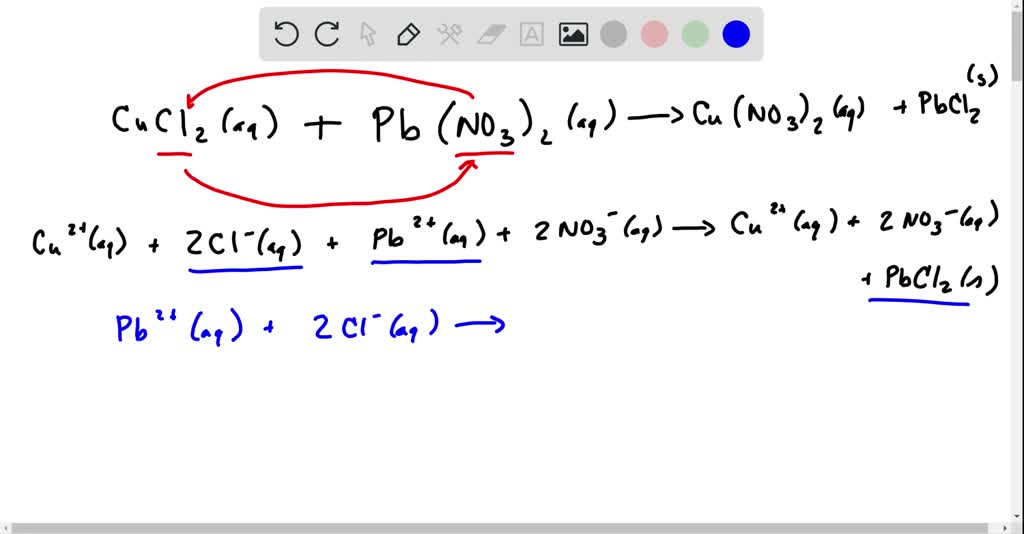
⏩SOLVEDCopper(Il) chloride and lead(II) nitrate react in aqueous
Copper Ii Nitrate Dissolved In Water Equation An aqueous solution is water that contains one or more dissolved substance. Copper is a much better reducing agent than water: This reaction is a cheap source of. Copper (ii) nitrate, often recognized by its chemical formula cu(no 3) 2, is an inorganic compound that has significant utility in a variety of industries and. The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility, defined as the maximum. 133 rows solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the rate. 2 cu (no 3) 2 → 2 cuo + 4 no 2 + o 2. An aqueous solution is water that contains one or more dissolved substance. In this chapter, we will focus on solution where the solvent is water. $$ \ce{3cu (s) + 2no3. Decomposition of copper nitrate yields nitrogen dioxide gas.
From www.numerade.com
SOLVED 'QUESTION 9 potassium nitrate, KNO3 dissolves water, H2O, the Copper Ii Nitrate Dissolved In Water Equation $$ \ce{3cu (s) + 2no3. The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility, defined as the maximum. This reaction is a cheap source of. 2 cu (no 3) 2 → 2 cuo + 4 no 2 + o 2. Copper (ii) nitrate, often recognized by its chemical formula. Copper Ii Nitrate Dissolved In Water Equation.
From byjus.com
Copper reacts with dilute nitric acid and forms copper nitrate, nitric Copper Ii Nitrate Dissolved In Water Equation Copper is a much better reducing agent than water: $$ \ce{3cu (s) + 2no3. Copper (ii) nitrate, often recognized by its chemical formula cu(no 3) 2, is an inorganic compound that has significant utility in a variety of industries and. An aqueous solution is water that contains one or more dissolved substance. This reaction is a cheap source of. 133. Copper Ii Nitrate Dissolved In Water Equation.
From www.coursehero.com
[Solved] Please see below question V The names and chemical formulae of Copper Ii Nitrate Dissolved In Water Equation In this chapter, we will focus on solution where the solvent is water. Copper is a much better reducing agent than water: 2 cu (no 3) 2 → 2 cuo + 4 no 2 + o 2. Copper (ii) nitrate, often recognized by its chemical formula cu(no 3) 2, is an inorganic compound that has significant utility in a variety. Copper Ii Nitrate Dissolved In Water Equation.
From www.coursehero.com
[Solved] Suppose 0.0615g of copper(II) nitrate is dissolved in 50.mL of Copper Ii Nitrate Dissolved In Water Equation 133 rows solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the rate. An aqueous solution is water that contains one or more dissolved substance. In this chapter, we will focus on solution where the solvent is water. $$ \ce{3cu (s) + 2no3. Copper (ii) nitrate, often recognized by its. Copper Ii Nitrate Dissolved In Water Equation.
From www.chegg.com
Solved What is the net ionic equation for the reaction of Copper Ii Nitrate Dissolved In Water Equation In this chapter, we will focus on solution where the solvent is water. Copper is a much better reducing agent than water: Decomposition of copper nitrate yields nitrogen dioxide gas. The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility, defined as the maximum. $$ \ce{3cu (s) + 2no3. Copper. Copper Ii Nitrate Dissolved In Water Equation.
From www.coursehero.com
[Solved] Suppose 0.0615g of copper(II) nitrate is dissolved in 50.mL of Copper Ii Nitrate Dissolved In Water Equation In this chapter, we will focus on solution where the solvent is water. 2 cu (no 3) 2 → 2 cuo + 4 no 2 + o 2. This reaction is a cheap source of. Copper is a much better reducing agent than water: An aqueous solution is water that contains one or more dissolved substance. $$ \ce{3cu (s) +. Copper Ii Nitrate Dissolved In Water Equation.
From www.chegg.com
Solved 3. Copper(II) nitrate reacts with potassium hydroxide Copper Ii Nitrate Dissolved In Water Equation Copper is a much better reducing agent than water: An aqueous solution is water that contains one or more dissolved substance. 2 cu (no 3) 2 → 2 cuo + 4 no 2 + o 2. In this chapter, we will focus on solution where the solvent is water. Copper (ii) nitrate, often recognized by its chemical formula cu(no 3). Copper Ii Nitrate Dissolved In Water Equation.
From www.toppr.com
On heating blue coloured powder of copper (II) nitrate in a boiling Copper Ii Nitrate Dissolved In Water Equation Copper is a much better reducing agent than water: Copper (ii) nitrate, often recognized by its chemical formula cu(no 3) 2, is an inorganic compound that has significant utility in a variety of industries and. The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility, defined as the maximum. In. Copper Ii Nitrate Dissolved In Water Equation.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Copper Ii Nitrate Dissolved In Water Equation In this chapter, we will focus on solution where the solvent is water. 2 cu (no 3) 2 → 2 cuo + 4 no 2 + o 2. 133 rows solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the rate. $$ \ce{3cu (s) + 2no3. Copper (ii) nitrate, often. Copper Ii Nitrate Dissolved In Water Equation.
From www.youtube.com
Equation for AgNO3 + H2O (Silver nitrate + Water) YouTube Copper Ii Nitrate Dissolved In Water Equation This reaction is a cheap source of. 133 rows solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the rate. 2 cu (no 3) 2 → 2 cuo + 4 no 2 + o 2. The extent to which a substance may be dissolved in water, or any solvent, is. Copper Ii Nitrate Dissolved In Water Equation.
From socratic.org
What are the products of the reaction between aqueous solutions of Copper Ii Nitrate Dissolved In Water Equation Decomposition of copper nitrate yields nitrogen dioxide gas. 2 cu (no 3) 2 → 2 cuo + 4 no 2 + o 2. The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility, defined as the maximum. $$ \ce{3cu (s) + 2no3. Copper is a much better reducing agent than. Copper Ii Nitrate Dissolved In Water Equation.
From waterdefense.org
How to Test Nitrates in Water (StepbyStep) Copper Ii Nitrate Dissolved In Water Equation 133 rows solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the rate. 2 cu (no 3) 2 → 2 cuo + 4 no 2 + o 2. Decomposition of copper nitrate yields nitrogen dioxide gas. This reaction is a cheap source of. Copper (ii) nitrate, often recognized by its. Copper Ii Nitrate Dissolved In Water Equation.
From pressbooks.pub
4.3 AcidBase Reactions Introduction to Chemistry Copper Ii Nitrate Dissolved In Water Equation Copper is a much better reducing agent than water: 133 rows solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the rate. In this chapter, we will focus on solution where the solvent is water. The extent to which a substance may be dissolved in water, or any solvent, is. Copper Ii Nitrate Dissolved In Water Equation.
From lab.honeywell.com
Copper(II) nitrate hemi(pentahydrate) 12837 Honeywell Research Copper Ii Nitrate Dissolved In Water Equation 133 rows solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the rate. 2 cu (no 3) 2 → 2 cuo + 4 no 2 + o 2. The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility, defined as. Copper Ii Nitrate Dissolved In Water Equation.
From www.numerade.com
SOLVED Write balanced chemical equations, including states of matter Copper Ii Nitrate Dissolved In Water Equation 133 rows solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the rate. In this chapter, we will focus on solution where the solvent is water. Copper is a much better reducing agent than water: An aqueous solution is water that contains one or more dissolved substance. This reaction is. Copper Ii Nitrate Dissolved In Water Equation.
From www.coursehero.com
[Solved] Reaction BCopper(II) Nitrate to Copper(II) Hydroxide Copper Ii Nitrate Dissolved In Water Equation In this chapter, we will focus on solution where the solvent is water. The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility, defined as the maximum. An aqueous solution is water that contains one or more dissolved substance. Copper (ii) nitrate, often recognized by its chemical formula cu(no 3). Copper Ii Nitrate Dissolved In Water Equation.
From www.youtube.com
Write the balanced chemical equation of the following word equation Copper Ii Nitrate Dissolved In Water Equation Copper is a much better reducing agent than water: 2 cu (no 3) 2 → 2 cuo + 4 no 2 + o 2. An aqueous solution is water that contains one or more dissolved substance. $$ \ce{3cu (s) + 2no3. Copper (ii) nitrate, often recognized by its chemical formula cu(no 3) 2, is an inorganic compound that has significant. Copper Ii Nitrate Dissolved In Water Equation.
From www.slideserve.com
PPT SURVEY OF CHEMISTRY LABORATORY I CHEM 1151L REACTIONS OF COPPER Copper Ii Nitrate Dissolved In Water Equation Copper (ii) nitrate, often recognized by its chemical formula cu(no 3) 2, is an inorganic compound that has significant utility in a variety of industries and. 2 cu (no 3) 2 → 2 cuo + 4 no 2 + o 2. $$ \ce{3cu (s) + 2no3. An aqueous solution is water that contains one or more dissolved substance. Decomposition of. Copper Ii Nitrate Dissolved In Water Equation.
From bryan-chapter.blogspot.com
Copper Nitrate And Sodium Hydroxide Equation 33+ Pages Summary [800kb Copper Ii Nitrate Dissolved In Water Equation Decomposition of copper nitrate yields nitrogen dioxide gas. 2 cu (no 3) 2 → 2 cuo + 4 no 2 + o 2. An aqueous solution is water that contains one or more dissolved substance. This reaction is a cheap source of. $$ \ce{3cu (s) + 2no3. In this chapter, we will focus on solution where the solvent is water.. Copper Ii Nitrate Dissolved In Water Equation.
From www.slideserve.com
PPT Preparation of silver nitrate and its uses PowerPoint Copper Ii Nitrate Dissolved In Water Equation Decomposition of copper nitrate yields nitrogen dioxide gas. 2 cu (no 3) 2 → 2 cuo + 4 no 2 + o 2. The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility, defined as the maximum. 133 rows solubility equilibrium defines the dynamic equilibria between a precipitate and its. Copper Ii Nitrate Dissolved In Water Equation.
From www.researchgate.net
Amount of copper nitrate in grams dissolved in 100 g of water and Copper Ii Nitrate Dissolved In Water Equation Copper (ii) nitrate, often recognized by its chemical formula cu(no 3) 2, is an inorganic compound that has significant utility in a variety of industries and. Decomposition of copper nitrate yields nitrogen dioxide gas. 133 rows solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the rate. $$ \ce{3cu (s). Copper Ii Nitrate Dissolved In Water Equation.
From giovani-khatfield.blogspot.com
What Is the Chemical Formula for Copper I Nitrite Copper Ii Nitrate Dissolved In Water Equation An aqueous solution is water that contains one or more dissolved substance. This reaction is a cheap source of. $$ \ce{3cu (s) + 2no3. Decomposition of copper nitrate yields nitrogen dioxide gas. Copper is a much better reducing agent than water: 133 rows solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of. Copper Ii Nitrate Dissolved In Water Equation.
From www.youtube.com
IGCSE Chemistry lesson 34 part c Thermal of nitrates Copper Ii Nitrate Dissolved In Water Equation Copper is a much better reducing agent than water: In this chapter, we will focus on solution where the solvent is water. An aqueous solution is water that contains one or more dissolved substance. This reaction is a cheap source of. 133 rows solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of. Copper Ii Nitrate Dissolved In Water Equation.
From www.numerade.com
SOLVED Write the word equations for each of the following chemical Copper Ii Nitrate Dissolved In Water Equation Copper is a much better reducing agent than water: Decomposition of copper nitrate yields nitrogen dioxide gas. 2 cu (no 3) 2 → 2 cuo + 4 no 2 + o 2. An aqueous solution is water that contains one or more dissolved substance. The extent to which a substance may be dissolved in water, or any solvent, is quantitatively. Copper Ii Nitrate Dissolved In Water Equation.
From www.transtutors.com
(Solved) Which Of The Following Is The Correct Dissociation Equation Copper Ii Nitrate Dissolved In Water Equation 133 rows solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the rate. This reaction is a cheap source of. Decomposition of copper nitrate yields nitrogen dioxide gas. Copper (ii) nitrate, often recognized by its chemical formula cu(no 3) 2, is an inorganic compound that has significant utility in a. Copper Ii Nitrate Dissolved In Water Equation.
From www.goldhilleducation.co.uk
IGCSE Chemistry Mychem Copper Ii Nitrate Dissolved In Water Equation This reaction is a cheap source of. The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility, defined as the maximum. $$ \ce{3cu (s) + 2no3. 133 rows solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the rate. An. Copper Ii Nitrate Dissolved In Water Equation.
From www.tessshebaylo.com
Net Ionic Equation Ammonium Bromide Dissolved In Water Tessshebaylo Copper Ii Nitrate Dissolved In Water Equation Copper is a much better reducing agent than water: The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility, defined as the maximum. $$ \ce{3cu (s) + 2no3. This reaction is a cheap source of. 2 cu (no 3) 2 → 2 cuo + 4 no 2 + o 2.. Copper Ii Nitrate Dissolved In Water Equation.
From www.youtube.com
How to Balance NaNO3 = NaNO2 + O2 of Sodium nitrate Copper Ii Nitrate Dissolved In Water Equation $$ \ce{3cu (s) + 2no3. The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility, defined as the maximum. 133 rows solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the rate. This reaction is a cheap source of. Decomposition. Copper Ii Nitrate Dissolved In Water Equation.
From www.numerade.com
SOLVED When copper(II) oxide reacts with dilute nitric acid, copper(II Copper Ii Nitrate Dissolved In Water Equation Copper (ii) nitrate, often recognized by its chemical formula cu(no 3) 2, is an inorganic compound that has significant utility in a variety of industries and. Decomposition of copper nitrate yields nitrogen dioxide gas. An aqueous solution is water that contains one or more dissolved substance. This reaction is a cheap source of. In this chapter, we will focus on. Copper Ii Nitrate Dissolved In Water Equation.
From brainly.in
The formula for hydrated copper(II) nitrate is Cu(NO3)2.XH20. It Copper Ii Nitrate Dissolved In Water Equation 133 rows solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the rate. The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility, defined as the maximum. Copper is a much better reducing agent than water: Decomposition of copper nitrate. Copper Ii Nitrate Dissolved In Water Equation.
From mungfali.com
Solved 1. Aqueous Ammonia Solution And Copper (ii) Nitrate 306 Copper Ii Nitrate Dissolved In Water Equation Decomposition of copper nitrate yields nitrogen dioxide gas. 133 rows solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the rate. An aqueous solution is water that contains one or more dissolved substance. $$ \ce{3cu (s) + 2no3. This reaction is a cheap source of. In this chapter, we will. Copper Ii Nitrate Dissolved In Water Equation.
From www.numerade.com
⏩SOLVEDCopper(Il) chloride and lead(II) nitrate react in aqueous Copper Ii Nitrate Dissolved In Water Equation The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility, defined as the maximum. In this chapter, we will focus on solution where the solvent is water. 2 cu (no 3) 2 → 2 cuo + 4 no 2 + o 2. $$ \ce{3cu (s) + 2no3. An aqueous solution. Copper Ii Nitrate Dissolved In Water Equation.
From brainly.com
Copper (II) nitrate, Cu(NO3)2, solution reacts with potassium hydroxide Copper Ii Nitrate Dissolved In Water Equation Decomposition of copper nitrate yields nitrogen dioxide gas. $$ \ce{3cu (s) + 2no3. Copper is a much better reducing agent than water: The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility, defined as the maximum. This reaction is a cheap source of. 133 rows solubility equilibrium defines the dynamic. Copper Ii Nitrate Dissolved In Water Equation.
From www.numerade.com
SOLVED A sample of copper (II) nitrate hydrate has a mass of 1.278 g Copper Ii Nitrate Dissolved In Water Equation The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility, defined as the maximum. $$ \ce{3cu (s) + 2no3. Copper (ii) nitrate, often recognized by its chemical formula cu(no 3) 2, is an inorganic compound that has significant utility in a variety of industries and. 2 cu (no 3) 2. Copper Ii Nitrate Dissolved In Water Equation.
From www.toppr.com
On heating blue coloured powder of copper (II) nitrate in a boiling Copper Ii Nitrate Dissolved In Water Equation Decomposition of copper nitrate yields nitrogen dioxide gas. The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility, defined as the maximum. 2 cu (no 3) 2 → 2 cuo + 4 no 2 + o 2. Copper is a much better reducing agent than water: 133 rows solubility equilibrium. Copper Ii Nitrate Dissolved In Water Equation.