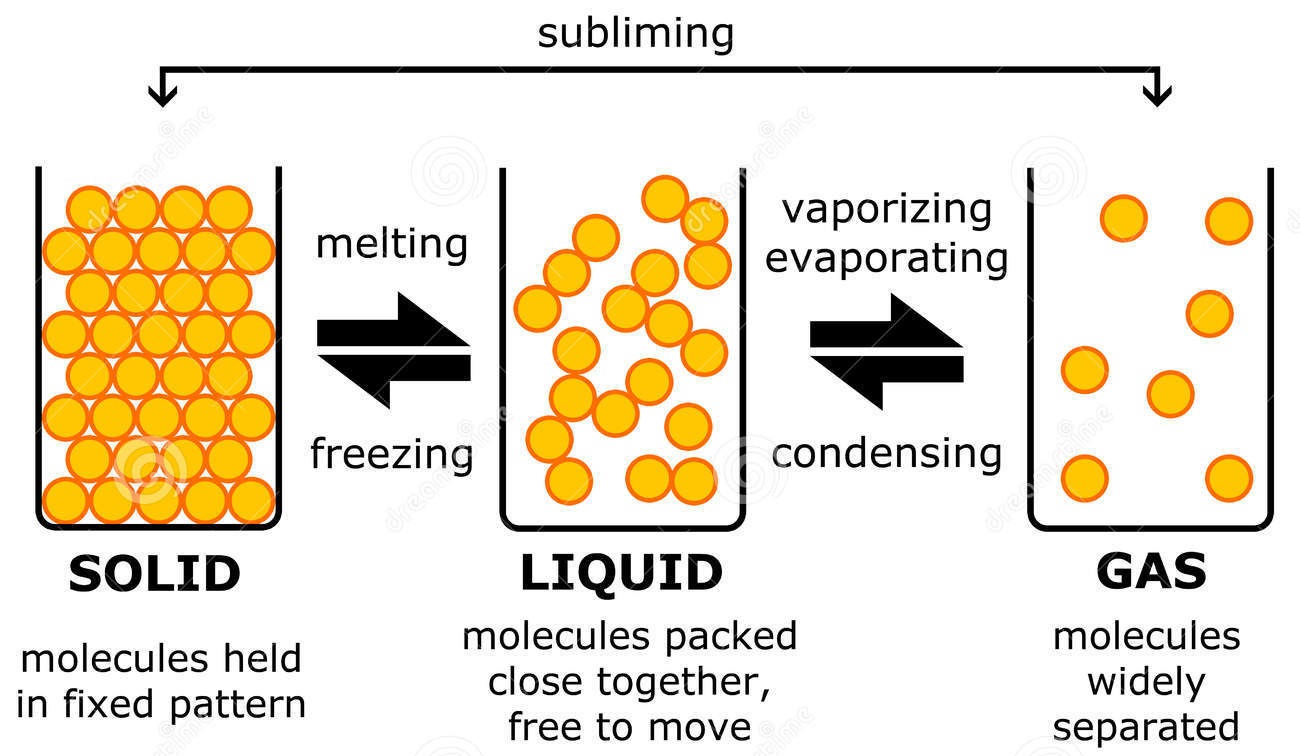
Solid Liquid Phase Change . The phase change from solid to gas is. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. The solid and liquid regions are separated by the. For water, there is no liquid phase at pressures below 0.00600 atm. Melting, freezing, evaporating, condensing, sublimination, and deposition (2). To calculate the energy changes that accompany phase changes. At sufficiently low pressures there is no liquid phase, but the substance can exist as either gas or solid. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool. There are six ways a substance can change between these three phases;
from www.radixtree.com
At sufficiently low pressures there is no liquid phase, but the substance can exist as either gas or solid. The phase change from solid to gas is. To calculate the energy changes that accompany phase changes. Melting, freezing, evaporating, condensing, sublimination, and deposition (2). The solid and liquid regions are separated by the. For water, there is no liquid phase at pressures below 0.00600 atm. There are six ways a substance can change between these three phases; Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool.
Physics Matter Online Education System
Solid Liquid Phase Change To calculate the energy changes that accompany phase changes. The solid and liquid regions are separated by the. There are six ways a substance can change between these three phases; Melting, freezing, evaporating, condensing, sublimination, and deposition (2). The phase change from solid to gas is. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. At sufficiently low pressures there is no liquid phase, but the substance can exist as either gas or solid. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool. For water, there is no liquid phase at pressures below 0.00600 atm. To calculate the energy changes that accompany phase changes.
From sciencenotes.org
Phase Changes of Matter (Phase Transitions) Solid Liquid Phase Change The phase change from solid to gas is. For water, there is no liquid phase at pressures below 0.00600 atm. Melting, freezing, evaporating, condensing, sublimination, and deposition (2). We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool. The solid and liquid regions are separated by the.. Solid Liquid Phase Change.
From www.dreamstime.com
States Matter Change Diagram Stock Illustrations 42 States Matter Solid Liquid Phase Change Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. At sufficiently low pressures there is no liquid phase, but the substance can exist as either gas or solid. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool. Melting, freezing, evaporating, condensing, sublimination,. Solid Liquid Phase Change.
From sciencenotes.org
States of Matter Solid Liquid Phase Change To calculate the energy changes that accompany phase changes. The solid and liquid regions are separated by the. At sufficiently low pressures there is no liquid phase, but the substance can exist as either gas or solid. There are six ways a substance can change between these three phases; We take advantage of changes between the gas, liquid, and solid. Solid Liquid Phase Change.
From www.worksheetsplanet.com
Phase Changes of Matter Solid Liquid Phase Change To calculate the energy changes that accompany phase changes. At sufficiently low pressures there is no liquid phase, but the substance can exist as either gas or solid. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. For water, there is no liquid phase at pressures below 0.00600 atm. There are six ways a substance can. Solid Liquid Phase Change.
From itinerantmission.blogspot.com
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas Solid Liquid Phase Change The phase change from solid to gas is. For water, there is no liquid phase at pressures below 0.00600 atm. To calculate the energy changes that accompany phase changes. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. There are six ways a substance can change between these three phases; We take advantage of changes between. Solid Liquid Phase Change.
From shaunmwilliams.com
Chapter 1 Presentation Solid Liquid Phase Change There are six ways a substance can change between these three phases; The solid and liquid regions are separated by the. To calculate the energy changes that accompany phase changes. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool. At sufficiently low pressures there is no. Solid Liquid Phase Change.
From sciencenotes.org
States of Matter Solid Liquid Phase Change There are six ways a substance can change between these three phases; To calculate the energy changes that accompany phase changes. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. For water, there is no liquid phase at pressures below 0.00600 atm. The phase change from solid to gas is. The solid and liquid regions are. Solid Liquid Phase Change.
From ny1.com
The amount of water on Earth is a constant Solid Liquid Phase Change At sufficiently low pressures there is no liquid phase, but the substance can exist as either gas or solid. The solid and liquid regions are separated by the. To calculate the energy changes that accompany phase changes. For water, there is no liquid phase at pressures below 0.00600 atm. We take advantage of changes between the gas, liquid, and solid. Solid Liquid Phase Change.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Solid Liquid Phase Change To calculate the energy changes that accompany phase changes. The phase change from solid to gas is. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool. At sufficiently low pressures there is no. Solid Liquid Phase Change.
From www.theschoolrun.com
What are states of matter? TheSchoolRun Solid Liquid Phase Change For water, there is no liquid phase at pressures below 0.00600 atm. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. There are six ways a substance can change between these three phases; We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool.. Solid Liquid Phase Change.
From www.snexplores.org
Explainer What are the different states of matter? Solid Liquid Phase Change Melting, freezing, evaporating, condensing, sublimination, and deposition (2). To calculate the energy changes that accompany phase changes. There are six ways a substance can change between these three phases; The solid and liquid regions are separated by the. The phase change from solid to gas is. At sufficiently low pressures there is no liquid phase, but the substance can exist. Solid Liquid Phase Change.
From chem.libretexts.org
5.6 Phase Diagrams Chemistry LibreTexts Solid Liquid Phase Change The phase change from solid to gas is. At sufficiently low pressures there is no liquid phase, but the substance can exist as either gas or solid. The solid and liquid regions are separated by the. Melting, freezing, evaporating, condensing, sublimination, and deposition (2). There are six ways a substance can change between these three phases; To calculate the energy. Solid Liquid Phase Change.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids Solid Liquid Phase Change The solid and liquid regions are separated by the. For water, there is no liquid phase at pressures below 0.00600 atm. To calculate the energy changes that accompany phase changes. There are six ways a substance can change between these three phases; Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. Melting, freezing, evaporating, condensing, sublimination,. Solid Liquid Phase Change.
From www.pinterest.com
Properties of Matter Phase Changes Shmoop Science diagrams Solid Liquid Phase Change We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool. At sufficiently low pressures there is no liquid phase, but the substance can exist as either gas or solid. The phase change from solid to gas is. The solid and liquid regions are separated by the. To. Solid Liquid Phase Change.
From www.thoughtco.com
List of Phase Changes Between States of Matter Solid Liquid Phase Change We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool. The phase change from solid to gas is. The solid and liquid regions are separated by the. To calculate the energy changes that accompany phase changes. Phase diagrams contain discrete regions corresponding to the solid, liquid, and. Solid Liquid Phase Change.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii Solid Liquid Phase Change To calculate the energy changes that accompany phase changes. There are six ways a substance can change between these three phases; For water, there is no liquid phase at pressures below 0.00600 atm. The solid and liquid regions are separated by the. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. At sufficiently low pressures there. Solid Liquid Phase Change.
From www.freepik.com
Premium Vector Water States of matter Phase Change of State for Water Solid Liquid Phase Change For water, there is no liquid phase at pressures below 0.00600 atm. At sufficiently low pressures there is no liquid phase, but the substance can exist as either gas or solid. There are six ways a substance can change between these three phases; We take advantage of changes between the gas, liquid, and solid states to cool a drink with. Solid Liquid Phase Change.
From courses.lumenlearning.com
Solid to Gas Phase Transition Introduction to Chemistry Solid Liquid Phase Change Melting, freezing, evaporating, condensing, sublimination, and deposition (2). For water, there is no liquid phase at pressures below 0.00600 atm. At sufficiently low pressures there is no liquid phase, but the substance can exist as either gas or solid. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. We take advantage of changes between the gas,. Solid Liquid Phase Change.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk Solid Liquid Phase Change The solid and liquid regions are separated by the. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. There are six ways a substance can change between these three phases; We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool. The phase change. Solid Liquid Phase Change.
From stock.adobe.com
Phase change transition diagram. States matter schema. Evaporation Solid Liquid Phase Change We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool. To calculate the energy changes that accompany phase changes. The solid and liquid regions are separated by the. For water, there is no liquid phase at pressures below 0.00600 atm. There are six ways a substance can. Solid Liquid Phase Change.
From www.vectorstock.com
Phase transitions of matter in water Royalty Free Vector Solid Liquid Phase Change For water, there is no liquid phase at pressures below 0.00600 atm. To calculate the energy changes that accompany phase changes. There are six ways a substance can change between these three phases; The solid and liquid regions are separated by the. Melting, freezing, evaporating, condensing, sublimination, and deposition (2). At sufficiently low pressures there is no liquid phase, but. Solid Liquid Phase Change.
From www.radixtree.com
Physics Matter Online Education System Solid Liquid Phase Change We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool. At sufficiently low pressures there is no liquid phase, but the substance can exist as either gas or solid. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. There are six ways a. Solid Liquid Phase Change.
From general.chemistrysteps.com
Entropy and State Change Chemistry Steps Solid Liquid Phase Change Melting, freezing, evaporating, condensing, sublimination, and deposition (2). The solid and liquid regions are separated by the. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool. At sufficiently low pressures there is no. Solid Liquid Phase Change.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Solid Liquid Phase Change For water, there is no liquid phase at pressures below 0.00600 atm. There are six ways a substance can change between these three phases; Melting, freezing, evaporating, condensing, sublimination, and deposition (2). At sufficiently low pressures there is no liquid phase, but the substance can exist as either gas or solid. The phase change from solid to gas is. To. Solid Liquid Phase Change.
From seonegativo.com
Cambio De Solido A Liquido Solid Liquid Phase Change The phase change from solid to gas is. At sufficiently low pressures there is no liquid phase, but the substance can exist as either gas or solid. The solid and liquid regions are separated by the. There are six ways a substance can change between these three phases; For water, there is no liquid phase at pressures below 0.00600 atm.. Solid Liquid Phase Change.
From courses.lumenlearning.com
Phase Diagrams Chemistry for Majors Solid Liquid Phase Change At sufficiently low pressures there is no liquid phase, but the substance can exist as either gas or solid. Melting, freezing, evaporating, condensing, sublimination, and deposition (2). The solid and liquid regions are separated by the. The phase change from solid to gas is. To calculate the energy changes that accompany phase changes. Phase diagrams contain discrete regions corresponding to. Solid Liquid Phase Change.
From www.reddit.com
ELI5 How can ice and liquid water be the same temperature? r Solid Liquid Phase Change The phase change from solid to gas is. The solid and liquid regions are separated by the. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool. For water, there is no liquid phase at pressures below 0.00600 atm. Melting, freezing, evaporating, condensing, sublimination, and deposition (2).. Solid Liquid Phase Change.
From www.thoughtco.com
List of Phase Changes Between States of Matter Solid Liquid Phase Change We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool. The phase change from solid to gas is. The solid and liquid regions are separated by the. Melting, freezing, evaporating, condensing, sublimination, and deposition (2). To calculate the energy changes that accompany phase changes. At sufficiently low. Solid Liquid Phase Change.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Solid Liquid Phase Change Melting, freezing, evaporating, condensing, sublimination, and deposition (2). For water, there is no liquid phase at pressures below 0.00600 atm. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. To calculate the energy. Solid Liquid Phase Change.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Solid Liquid Phase Change For water, there is no liquid phase at pressures below 0.00600 atm. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. There are six ways a substance can change between these three phases; To calculate the energy changes that accompany phase changes. The phase change from solid to gas is. The solid and liquid regions are. Solid Liquid Phase Change.
From emedia.rmit.edu.au
States of matter Learning Lab Solid Liquid Phase Change For water, there is no liquid phase at pressures below 0.00600 atm. There are six ways a substance can change between these three phases; The solid and liquid regions are separated by the. At sufficiently low pressures there is no liquid phase, but the substance can exist as either gas or solid. To calculate the energy changes that accompany phase. Solid Liquid Phase Change.
From kids.britannica.com
solid Students Britannica Kids Homework Help Solid Liquid Phase Change The solid and liquid regions are separated by the. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool. The phase change from solid to gas is. At sufficiently low pressures there is no. Solid Liquid Phase Change.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Solid Liquid Phase Change The phase change from solid to gas is. To calculate the energy changes that accompany phase changes. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. Melting, freezing, evaporating, condensing, sublimination, and deposition. Solid Liquid Phase Change.
From www.britannica.com
phase Definition & Facts Britannica Solid Liquid Phase Change The phase change from solid to gas is. For water, there is no liquid phase at pressures below 0.00600 atm. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. There are six ways a substance can change between these three phases; Melting, freezing, evaporating, condensing, sublimination, and deposition (2). To calculate the energy changes that accompany. Solid Liquid Phase Change.
From middleschoolscience.com
Solid, Liquid, & Gas Triple Venn Diagram Activity Middle School Solid Liquid Phase Change At sufficiently low pressures there is no liquid phase, but the substance can exist as either gas or solid. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool. The solid and liquid regions are separated by the. There are six ways a substance can change between. Solid Liquid Phase Change.