Zinc And Copper Chloride Reaction . This is a redox reaction in which the oxidation states of the metals clearly change. In the first reaction, the copper ion is able to oxidize the zinc metal. The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Zinc metal is oxidized to zn2+,. In this video we'll balance the equation zn + cucl2 = zncl2 + cu and provide the correct coefficients for each compound. However, in the second reaction, the zinc ion is not able to oxidize the. Today in class we did the gold penny experiment, where we put metallic zinc and a penny in a zinc chloride $\pu{1 m}$. We observe that silver colored zinc is added to blue colored copper (ii) chloride to reduce the copper (ii) ions into elemental copper.
from 2012books.lardbucket.org
Zinc metal is oxidized to zn2+,. Today in class we did the gold penny experiment, where we put metallic zinc and a penny in a zinc chloride $\pu{1 m}$. This is a redox reaction in which the oxidation states of the metals clearly change. In the first reaction, the copper ion is able to oxidize the zinc metal. However, in the second reaction, the zinc ion is not able to oxidize the. We observe that silver colored zinc is added to blue colored copper (ii) chloride to reduce the copper (ii) ions into elemental copper. The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. In this video we'll balance the equation zn + cucl2 = zncl2 + cu and provide the correct coefficients for each compound.
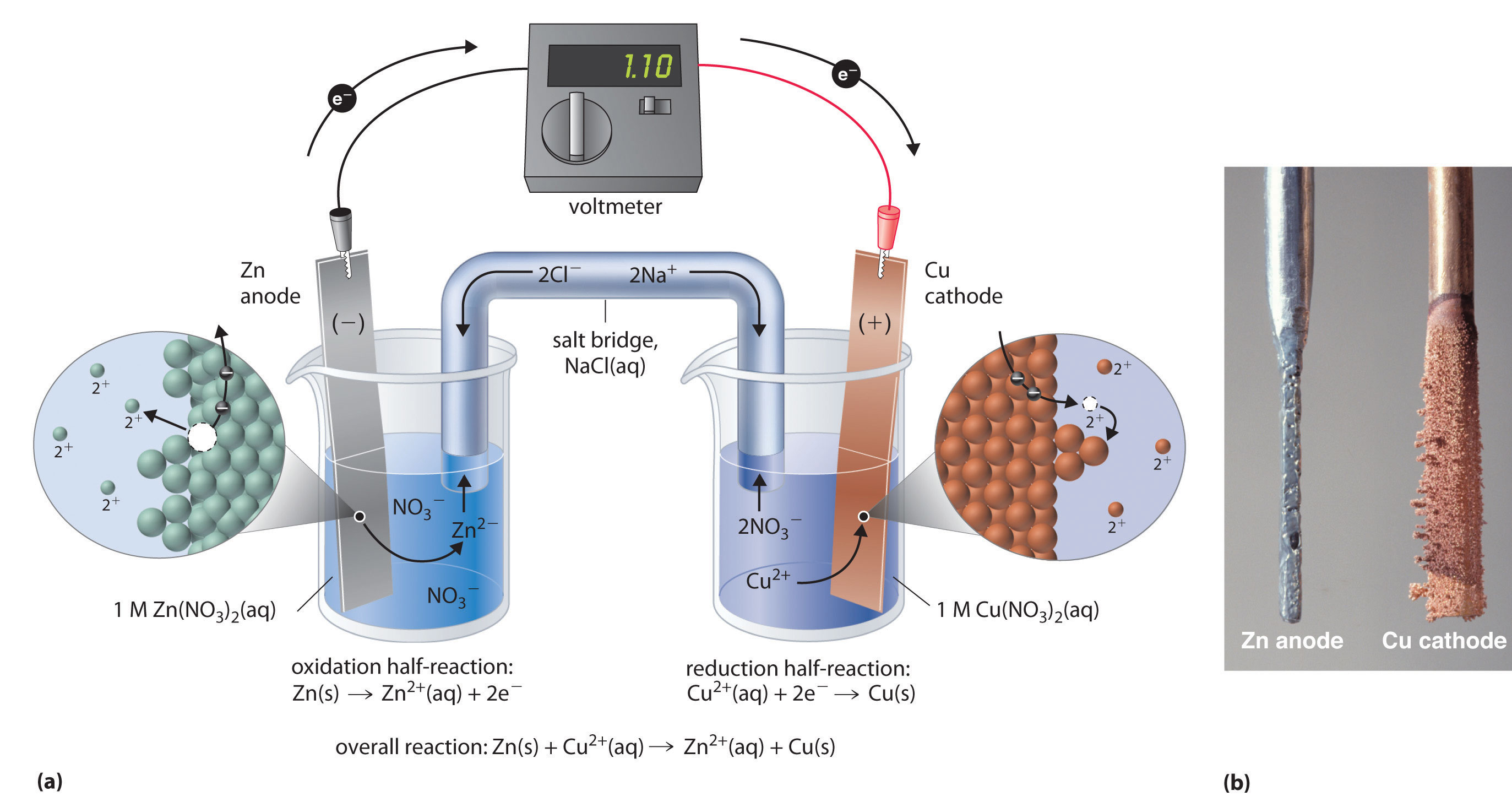
Describing Electrochemical Cells
Zinc And Copper Chloride Reaction The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Zinc metal is oxidized to zn2+,. In the first reaction, the copper ion is able to oxidize the zinc metal. This is a redox reaction in which the oxidation states of the metals clearly change. We observe that silver colored zinc is added to blue colored copper (ii) chloride to reduce the copper (ii) ions into elemental copper. In this video we'll balance the equation zn + cucl2 = zncl2 + cu and provide the correct coefficients for each compound. Today in class we did the gold penny experiment, where we put metallic zinc and a penny in a zinc chloride $\pu{1 m}$. However, in the second reaction, the zinc ion is not able to oxidize the. The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals.
From www.youtube.com
Redox reaction from dissolving zinc in copper sulfate Chemistry Zinc And Copper Chloride Reaction We observe that silver colored zinc is added to blue colored copper (ii) chloride to reduce the copper (ii) ions into elemental copper. Zinc metal is oxidized to zn2+,. However, in the second reaction, the zinc ion is not able to oxidize the. In the first reaction, the copper ion is able to oxidize the zinc metal. In this video. Zinc And Copper Chloride Reaction.
From express.adobe.com
Zinc and Copper Chloride Zinc And Copper Chloride Reaction In the first reaction, the copper ion is able to oxidize the zinc metal. Zinc metal is oxidized to zn2+,. We observe that silver colored zinc is added to blue colored copper (ii) chloride to reduce the copper (ii) ions into elemental copper. However, in the second reaction, the zinc ion is not able to oxidize the. The experiment reinforces. Zinc And Copper Chloride Reaction.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Zinc And Copper Chloride Reaction In the first reaction, the copper ion is able to oxidize the zinc metal. However, in the second reaction, the zinc ion is not able to oxidize the. We observe that silver colored zinc is added to blue colored copper (ii) chloride to reduce the copper (ii) ions into elemental copper. The experiment reinforces ideas about energy changes during reactions,. Zinc And Copper Chloride Reaction.
From www.coursehero.com
[Solved] link for the experiment https//youtu.be/ILJhI43wVA Part Zinc And Copper Chloride Reaction In the first reaction, the copper ion is able to oxidize the zinc metal. We observe that silver colored zinc is added to blue colored copper (ii) chloride to reduce the copper (ii) ions into elemental copper. This is a redox reaction in which the oxidation states of the metals clearly change. However, in the second reaction, the zinc ion. Zinc And Copper Chloride Reaction.
From express.adobe.com
Zinc and Copper Chloride Zinc And Copper Chloride Reaction The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. In the first reaction, the copper ion is able to oxidize the zinc metal. Today in class we did the gold penny experiment, where we put metallic zinc and a penny in a zinc chloride $\pu{1 m}$. Zinc metal. Zinc And Copper Chloride Reaction.
From flatworldknowledge.lardbucket.org
Describing Electrochemical Cells Zinc And Copper Chloride Reaction We observe that silver colored zinc is added to blue colored copper (ii) chloride to reduce the copper (ii) ions into elemental copper. Today in class we did the gold penny experiment, where we put metallic zinc and a penny in a zinc chloride $\pu{1 m}$. In the first reaction, the copper ion is able to oxidize the zinc metal.. Zinc And Copper Chloride Reaction.
From spark.adobe.com
Zinc and Copper Chloride Reaction Zinc And Copper Chloride Reaction Zinc metal is oxidized to zn2+,. In this video we'll balance the equation zn + cucl2 = zncl2 + cu and provide the correct coefficients for each compound. The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Today in class we did the gold penny experiment, where we. Zinc And Copper Chloride Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction copper zinc Fundamental Photographs Zinc And Copper Chloride Reaction In the first reaction, the copper ion is able to oxidize the zinc metal. This is a redox reaction in which the oxidation states of the metals clearly change. Zinc metal is oxidized to zn2+,. Today in class we did the gold penny experiment, where we put metallic zinc and a penny in a zinc chloride $\pu{1 m}$. However, in. Zinc And Copper Chloride Reaction.
From www.youtube.com
zinc and copper chloride react YouTube Zinc And Copper Chloride Reaction We observe that silver colored zinc is added to blue colored copper (ii) chloride to reduce the copper (ii) ions into elemental copper. However, in the second reaction, the zinc ion is not able to oxidize the. Today in class we did the gold penny experiment, where we put metallic zinc and a penny in a zinc chloride $\pu{1 m}$.. Zinc And Copper Chloride Reaction.
From spark.adobe.com
Zinc and Copper Chloride Zinc And Copper Chloride Reaction However, in the second reaction, the zinc ion is not able to oxidize the. This is a redox reaction in which the oxidation states of the metals clearly change. Zinc metal is oxidized to zn2+,. The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Today in class we. Zinc And Copper Chloride Reaction.
From www.chegg.com
Solved What is the net ionic equation for the reaction of Zinc And Copper Chloride Reaction We observe that silver colored zinc is added to blue colored copper (ii) chloride to reduce the copper (ii) ions into elemental copper. Zinc metal is oxidized to zn2+,. In this video we'll balance the equation zn + cucl2 = zncl2 + cu and provide the correct coefficients for each compound. In the first reaction, the copper ion is able. Zinc And Copper Chloride Reaction.
From express.adobe.com
Zinc and Copper Chloride Reaction Zinc And Copper Chloride Reaction Today in class we did the gold penny experiment, where we put metallic zinc and a penny in a zinc chloride $\pu{1 m}$. The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. We observe that silver colored zinc is added to blue colored copper (ii) chloride to reduce. Zinc And Copper Chloride Reaction.
From www.youtube.com
The Reaction of Zinc Metal and Aqueous Copper(II) YouTube Zinc And Copper Chloride Reaction However, in the second reaction, the zinc ion is not able to oxidize the. Zinc metal is oxidized to zn2+,. The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Today in class we did the gold penny experiment, where we put metallic zinc and a penny in a. Zinc And Copper Chloride Reaction.
From www.youtube.com
How to Write the Net Ionic Equation for CuCl2 + Zn = ZnCl2 + Cu YouTube Zinc And Copper Chloride Reaction However, in the second reaction, the zinc ion is not able to oxidize the. Zinc metal is oxidized to zn2+,. This is a redox reaction in which the oxidation states of the metals clearly change. Today in class we did the gold penny experiment, where we put metallic zinc and a penny in a zinc chloride $\pu{1 m}$. In this. Zinc And Copper Chloride Reaction.
From www.pw.live
Zinc Chloride Formula Zinc And Copper Chloride Reaction Zinc metal is oxidized to zn2+,. This is a redox reaction in which the oxidation states of the metals clearly change. In this video we'll balance the equation zn + cucl2 = zncl2 + cu and provide the correct coefficients for each compound. We observe that silver colored zinc is added to blue colored copper (ii) chloride to reduce the. Zinc And Copper Chloride Reaction.
From www.chegg.com
Solved 761. Zinc metal reacts with aqueous copper(II) Zinc And Copper Chloride Reaction The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. However, in the second reaction, the zinc ion is not able to oxidize the. Today in class we did the gold penny experiment, where we put metallic zinc and a penny in a zinc chloride $\pu{1 m}$. In the. Zinc And Copper Chloride Reaction.
From spark.adobe.com
Zinc and Copper Chloride Reaction Zinc And Copper Chloride Reaction In this video we'll balance the equation zn + cucl2 = zncl2 + cu and provide the correct coefficients for each compound. In the first reaction, the copper ion is able to oxidize the zinc metal. Today in class we did the gold penny experiment, where we put metallic zinc and a penny in a zinc chloride $\pu{1 m}$. This. Zinc And Copper Chloride Reaction.
From inchem.netlify.app
Reaction of zinc powder with cucl2 solution inchem Zinc And Copper Chloride Reaction We observe that silver colored zinc is added to blue colored copper (ii) chloride to reduce the copper (ii) ions into elemental copper. However, in the second reaction, the zinc ion is not able to oxidize the. In this video we'll balance the equation zn + cucl2 = zncl2 + cu and provide the correct coefficients for each compound. This. Zinc And Copper Chloride Reaction.
From www.youtube.com
Zinc + Copper Sulfate Reaction YouTube Zinc And Copper Chloride Reaction This is a redox reaction in which the oxidation states of the metals clearly change. In this video we'll balance the equation zn + cucl2 = zncl2 + cu and provide the correct coefficients for each compound. Zinc metal is oxidized to zn2+,. Today in class we did the gold penny experiment, where we put metallic zinc and a penny. Zinc And Copper Chloride Reaction.
From spmscience.blog.onlinetuition.com.my
Electrolysis of Copper (II) Chloride Solution SPM Science Zinc And Copper Chloride Reaction However, in the second reaction, the zinc ion is not able to oxidize the. Zinc metal is oxidized to zn2+,. In this video we'll balance the equation zn + cucl2 = zncl2 + cu and provide the correct coefficients for each compound. Today in class we did the gold penny experiment, where we put metallic zinc and a penny in. Zinc And Copper Chloride Reaction.
From www.youtube.com
zinc and copper chloride react YouTube Zinc And Copper Chloride Reaction In the first reaction, the copper ion is able to oxidize the zinc metal. Zinc metal is oxidized to zn2+,. However, in the second reaction, the zinc ion is not able to oxidize the. This is a redox reaction in which the oxidation states of the metals clearly change. The experiment reinforces ideas about energy changes during reactions, the reactivity. Zinc And Copper Chloride Reaction.
From lexiqosnow.blogspot.com
Balancing Net Ionic Redox Equations LexiqoSnow Zinc And Copper Chloride Reaction We observe that silver colored zinc is added to blue colored copper (ii) chloride to reduce the copper (ii) ions into elemental copper. The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Zinc metal is oxidized to zn2+,. This is a redox reaction in which the oxidation states. Zinc And Copper Chloride Reaction.
From express.adobe.com
Zinc and Copper Chloride Zinc And Copper Chloride Reaction Today in class we did the gold penny experiment, where we put metallic zinc and a penny in a zinc chloride $\pu{1 m}$. However, in the second reaction, the zinc ion is not able to oxidize the. Zinc metal is oxidized to zn2+,. We observe that silver colored zinc is added to blue colored copper (ii) chloride to reduce the. Zinc And Copper Chloride Reaction.
From www.youtube.com
How to Balance Zn + CuCl2 = ZnCl2 + Cu Zinc + Copper (II) chloride Zinc And Copper Chloride Reaction The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Zinc metal is oxidized to zn2+,. In the first reaction, the copper ion is able to oxidize the zinc metal. However, in the second reaction, the zinc ion is not able to oxidize the. Today in class we did. Zinc And Copper Chloride Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction zinc copper Fundamental Photographs Zinc And Copper Chloride Reaction In the first reaction, the copper ion is able to oxidize the zinc metal. We observe that silver colored zinc is added to blue colored copper (ii) chloride to reduce the copper (ii) ions into elemental copper. The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Zinc metal. Zinc And Copper Chloride Reaction.
From www.chegg.com
Solved Reaction 6 The reaction between zinc and copper (I) Zinc And Copper Chloride Reaction However, in the second reaction, the zinc ion is not able to oxidize the. In the first reaction, the copper ion is able to oxidize the zinc metal. In this video we'll balance the equation zn + cucl2 = zncl2 + cu and provide the correct coefficients for each compound. Zinc metal is oxidized to zn2+,. This is a redox. Zinc And Copper Chloride Reaction.
From brainly.com
Zinc+Copper chloride> is the reaction between zinc and copper chloride Zinc And Copper Chloride Reaction In this video we'll balance the equation zn + cucl2 = zncl2 + cu and provide the correct coefficients for each compound. This is a redox reaction in which the oxidation states of the metals clearly change. Today in class we did the gold penny experiment, where we put metallic zinc and a penny in a zinc chloride $\pu{1 m}$.. Zinc And Copper Chloride Reaction.
From 2012books.lardbucket.org
Describing Electrochemical Cells Zinc And Copper Chloride Reaction Zinc metal is oxidized to zn2+,. However, in the second reaction, the zinc ion is not able to oxidize the. In this video we'll balance the equation zn + cucl2 = zncl2 + cu and provide the correct coefficients for each compound. Today in class we did the gold penny experiment, where we put metallic zinc and a penny in. Zinc And Copper Chloride Reaction.
From www.youtube.com
copper chloride and zinc reaction YouTube Zinc And Copper Chloride Reaction In this video we'll balance the equation zn + cucl2 = zncl2 + cu and provide the correct coefficients for each compound. In the first reaction, the copper ion is able to oxidize the zinc metal. We observe that silver colored zinc is added to blue colored copper (ii) chloride to reduce the copper (ii) ions into elemental copper. Zinc. Zinc And Copper Chloride Reaction.
From spark.adobe.com
Zinc and Copper Chloride Reaction Zinc And Copper Chloride Reaction The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. This is a redox reaction in which the oxidation states of the metals clearly change. In this video we'll balance the equation zn + cucl2 = zncl2 + cu and provide the correct coefficients for each compound. Today in. Zinc And Copper Chloride Reaction.
From psu.pb.unizin.org
17.3 Standard Reduction Potentials Chemistry 112 Chapters 1217 of Zinc And Copper Chloride Reaction This is a redox reaction in which the oxidation states of the metals clearly change. The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. In this video we'll balance the equation zn + cucl2 = zncl2 + cu and provide the correct coefficients for each compound. However, in. Zinc And Copper Chloride Reaction.
From www.chegg.com
Solved B. Zinc and Copper(II) Chloride reactant 1 + reactant Zinc And Copper Chloride Reaction The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. In this video we'll balance the equation zn + cucl2 = zncl2 + cu and provide the correct coefficients for each compound. Today in class we did the gold penny experiment, where we put metallic zinc and a penny. Zinc And Copper Chloride Reaction.
From www.youtube.com
Zinc and Copper II Chloride YouTube Zinc And Copper Chloride Reaction We observe that silver colored zinc is added to blue colored copper (ii) chloride to reduce the copper (ii) ions into elemental copper. However, in the second reaction, the zinc ion is not able to oxidize the. In the first reaction, the copper ion is able to oxidize the zinc metal. Today in class we did the gold penny experiment,. Zinc And Copper Chloride Reaction.
From spark.adobe.com
Zinc and Copper Chloride Reaction Zinc And Copper Chloride Reaction Zinc metal is oxidized to zn2+,. We observe that silver colored zinc is added to blue colored copper (ii) chloride to reduce the copper (ii) ions into elemental copper. This is a redox reaction in which the oxidation states of the metals clearly change. In this video we'll balance the equation zn + cucl2 = zncl2 + cu and provide. Zinc And Copper Chloride Reaction.
From shaunmwilliams.com
Chapter 14 Presentation Zinc And Copper Chloride Reaction In this video we'll balance the equation zn + cucl2 = zncl2 + cu and provide the correct coefficients for each compound. We observe that silver colored zinc is added to blue colored copper (ii) chloride to reduce the copper (ii) ions into elemental copper. Zinc metal is oxidized to zn2+,. The experiment reinforces ideas about energy changes during reactions,. Zinc And Copper Chloride Reaction.