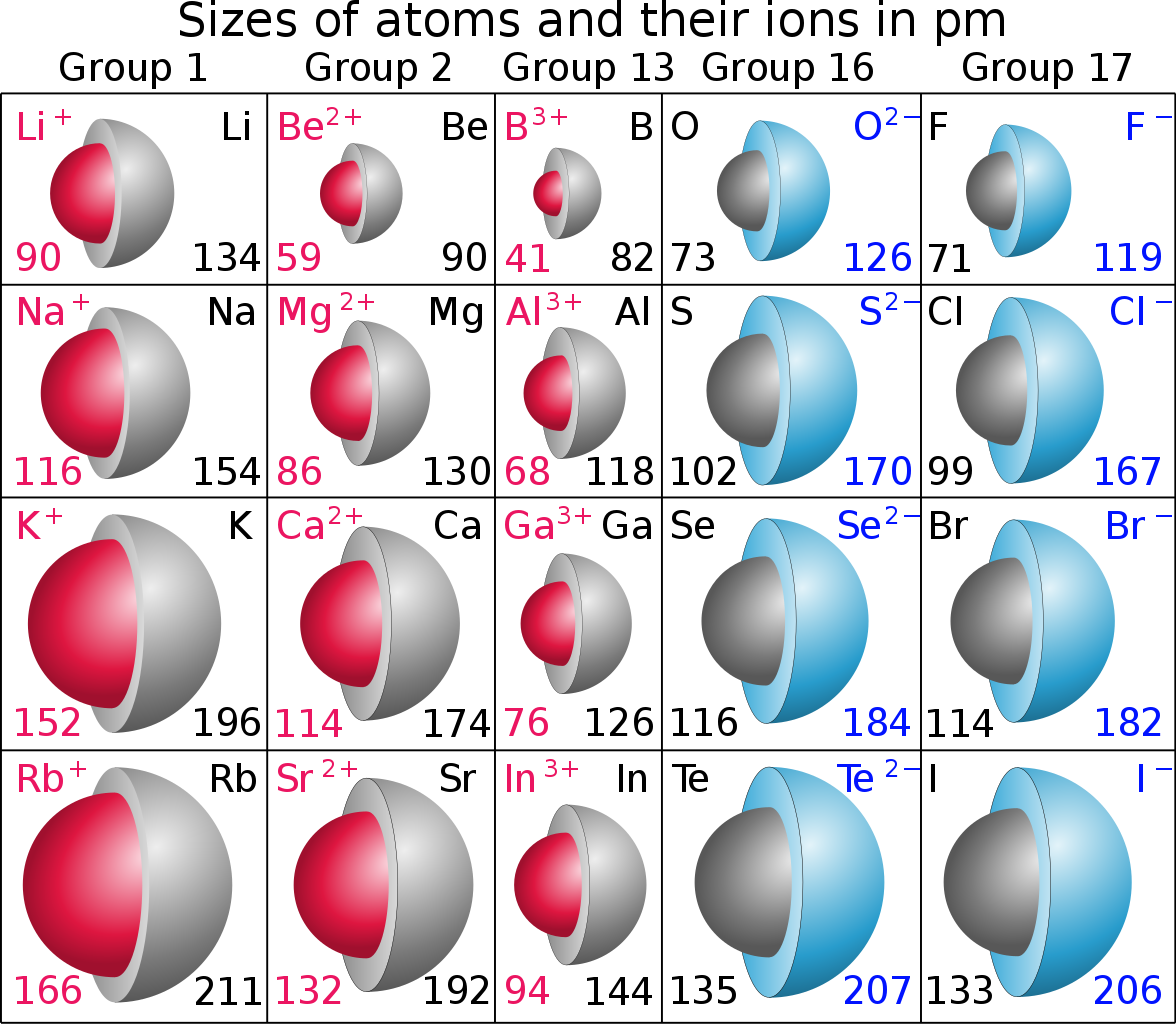
Radius Ratio And Geometry Of Ionic Compounds . Radius ratio rules in ionic solids: In order to derive the minimum. Radius ratio refers to as the ration of smaller ionic radius (cation) by the ratio of larger ionic radius (anion). This rule indicates that for an ionic compound to be stable, there is a critical range of radius ratios that can influence the coordination number, which. The model of radius ratio allows a prediction of the coordination number (cn) in an ionic compound. Radius ratio rule for ionic compounds •(1) in crystals, cations tend to get surrounded by the largest possible number of anions around it. The table below gives the ranges of cation/anion radius ratios that give the best fit for a given coordination geometry. Ionic model & ionic radius, definition of radius ratio, calculation of limiting radius ratio values, limitations, practice. Importance of the radius ratio rule Hence, radius ration ρ = r s /r l. The structure of many ionic solids can be accounted by considering the relative sizes of the cation and anion, and their relative numbers.
from socratic.org
Radius ratio refers to as the ration of smaller ionic radius (cation) by the ratio of larger ionic radius (anion). Radius ratio rules in ionic solids: Hence, radius ration ρ = r s /r l. The model of radius ratio allows a prediction of the coordination number (cn) in an ionic compound. The structure of many ionic solids can be accounted by considering the relative sizes of the cation and anion, and their relative numbers. Radius ratio rule for ionic compounds •(1) in crystals, cations tend to get surrounded by the largest possible number of anions around it. The table below gives the ranges of cation/anion radius ratios that give the best fit for a given coordination geometry. In order to derive the minimum. This rule indicates that for an ionic compound to be stable, there is a critical range of radius ratios that can influence the coordination number, which. Importance of the radius ratio rule
How does ionic radius change across a period? Socratic
Radius Ratio And Geometry Of Ionic Compounds Radius ratio rule for ionic compounds •(1) in crystals, cations tend to get surrounded by the largest possible number of anions around it. Radius ratio rule for ionic compounds •(1) in crystals, cations tend to get surrounded by the largest possible number of anions around it. The model of radius ratio allows a prediction of the coordination number (cn) in an ionic compound. In order to derive the minimum. This rule indicates that for an ionic compound to be stable, there is a critical range of radius ratios that can influence the coordination number, which. Hence, radius ration ρ = r s /r l. Importance of the radius ratio rule Ionic model & ionic radius, definition of radius ratio, calculation of limiting radius ratio values, limitations, practice. Radius ratio rules in ionic solids: Radius ratio refers to as the ration of smaller ionic radius (cation) by the ratio of larger ionic radius (anion). The structure of many ionic solids can be accounted by considering the relative sizes of the cation and anion, and their relative numbers. The table below gives the ranges of cation/anion radius ratios that give the best fit for a given coordination geometry.
From www.slideserve.com
PPT Chemistry periodicity Atomic and Ionic Radius PowerPoint Radius Ratio And Geometry Of Ionic Compounds Ionic model & ionic radius, definition of radius ratio, calculation of limiting radius ratio values, limitations, practice. The model of radius ratio allows a prediction of the coordination number (cn) in an ionic compound. The structure of many ionic solids can be accounted by considering the relative sizes of the cation and anion, and their relative numbers. Importance of the. Radius Ratio And Geometry Of Ionic Compounds.
From neetlab.com
Ionic Radius NEET Lab Radius Ratio And Geometry Of Ionic Compounds Importance of the radius ratio rule The table below gives the ranges of cation/anion radius ratios that give the best fit for a given coordination geometry. Radius ratio refers to as the ration of smaller ionic radius (cation) by the ratio of larger ionic radius (anion). Hence, radius ration ρ = r s /r l. The model of radius ratio. Radius Ratio And Geometry Of Ionic Compounds.
From byjus.com
is it necessary to remember the radius ratios of each specific ionic Radius Ratio And Geometry Of Ionic Compounds Importance of the radius ratio rule Radius ratio rules in ionic solids: Hence, radius ration ρ = r s /r l. Ionic model & ionic radius, definition of radius ratio, calculation of limiting radius ratio values, limitations, practice. In order to derive the minimum. This rule indicates that for an ionic compound to be stable, there is a critical range. Radius Ratio And Geometry Of Ionic Compounds.
From www.slideserve.com
PPT Naming Ionic Compounds PowerPoint Presentation, free download Radius Ratio And Geometry Of Ionic Compounds Importance of the radius ratio rule The model of radius ratio allows a prediction of the coordination number (cn) in an ionic compound. Ionic model & ionic radius, definition of radius ratio, calculation of limiting radius ratio values, limitations, practice. Radius ratio refers to as the ration of smaller ionic radius (cation) by the ratio of larger ionic radius (anion).. Radius Ratio And Geometry Of Ionic Compounds.
From sciencenotes.org
Atomic Radius and Ionic Radius Radius Ratio And Geometry Of Ionic Compounds The table below gives the ranges of cation/anion radius ratios that give the best fit for a given coordination geometry. Ionic model & ionic radius, definition of radius ratio, calculation of limiting radius ratio values, limitations, practice. This rule indicates that for an ionic compound to be stable, there is a critical range of radius ratios that can influence the. Radius Ratio And Geometry Of Ionic Compounds.
From www.youtube.com
Radius Ratio Rule & Structure of Ionic Compounds Class 12 Chemistry Radius Ratio And Geometry Of Ionic Compounds Hence, radius ration ρ = r s /r l. The structure of many ionic solids can be accounted by considering the relative sizes of the cation and anion, and their relative numbers. Radius ratio rule for ionic compounds •(1) in crystals, cations tend to get surrounded by the largest possible number of anions around it. In order to derive the. Radius Ratio And Geometry Of Ionic Compounds.
From chemistrytalk.org
Ionic Radius Trends ChemTalk Radius Ratio And Geometry Of Ionic Compounds The model of radius ratio allows a prediction of the coordination number (cn) in an ionic compound. Ionic model & ionic radius, definition of radius ratio, calculation of limiting radius ratio values, limitations, practice. Radius ratio refers to as the ration of smaller ionic radius (cation) by the ratio of larger ionic radius (anion). Radius ratio rules in ionic solids:. Radius Ratio And Geometry Of Ionic Compounds.
From www.slideserve.com
PPT Lecture 4 (Chapter 13 in Perkins) Crystal Chemistry Part 3 Radius Ratio And Geometry Of Ionic Compounds Hence, radius ration ρ = r s /r l. The table below gives the ranges of cation/anion radius ratios that give the best fit for a given coordination geometry. Ionic model & ionic radius, definition of radius ratio, calculation of limiting radius ratio values, limitations, practice. The structure of many ionic solids can be accounted by considering the relative sizes. Radius Ratio And Geometry Of Ionic Compounds.
From www.youtube.com
Radius Ratio Rule for Ionic compounds.. Radius Ratio Chart.. Chemical Radius Ratio And Geometry Of Ionic Compounds This rule indicates that for an ionic compound to be stable, there is a critical range of radius ratios that can influence the coordination number, which. Radius ratio rule for ionic compounds •(1) in crystals, cations tend to get surrounded by the largest possible number of anions around it. The table below gives the ranges of cation/anion radius ratios that. Radius Ratio And Geometry Of Ionic Compounds.
From www.chegg.com
Solved Briefly describe how the radius ratio rule can be Radius Ratio And Geometry Of Ionic Compounds Radius ratio refers to as the ration of smaller ionic radius (cation) by the ratio of larger ionic radius (anion). This rule indicates that for an ionic compound to be stable, there is a critical range of radius ratios that can influence the coordination number, which. In order to derive the minimum. Hence, radius ration ρ = r s /r. Radius Ratio And Geometry Of Ionic Compounds.
From www.slideserve.com
PPT SOLID STATE CHEMISTRY PowerPoint Presentation, free download ID Radius Ratio And Geometry Of Ionic Compounds Importance of the radius ratio rule The structure of many ionic solids can be accounted by considering the relative sizes of the cation and anion, and their relative numbers. Radius ratio refers to as the ration of smaller ionic radius (cation) by the ratio of larger ionic radius (anion). This rule indicates that for an ionic compound to be stable,. Radius Ratio And Geometry Of Ionic Compounds.
From byjus.com
The set representing the correct order of ionic radius is(A) Li+ > Be2 Radius Ratio And Geometry Of Ionic Compounds Radius ratio rules in ionic solids: This rule indicates that for an ionic compound to be stable, there is a critical range of radius ratios that can influence the coordination number, which. Importance of the radius ratio rule The table below gives the ranges of cation/anion radius ratios that give the best fit for a given coordination geometry. Radius ratio. Radius Ratio And Geometry Of Ionic Compounds.
From sciencenotes.org
Atomic Radius and Ionic Radius Radius Ratio And Geometry Of Ionic Compounds This rule indicates that for an ionic compound to be stable, there is a critical range of radius ratios that can influence the coordination number, which. In order to derive the minimum. Ionic model & ionic radius, definition of radius ratio, calculation of limiting radius ratio values, limitations, practice. Radius ratio refers to as the ration of smaller ionic radius. Radius Ratio And Geometry Of Ionic Compounds.
From utedzz.blogspot.com
Ionic Radius Periodic Table Trend Periodic Table Timeline Radius Ratio And Geometry Of Ionic Compounds This rule indicates that for an ionic compound to be stable, there is a critical range of radius ratios that can influence the coordination number, which. The model of radius ratio allows a prediction of the coordination number (cn) in an ionic compound. Radius ratio refers to as the ration of smaller ionic radius (cation) by the ratio of larger. Radius Ratio And Geometry Of Ionic Compounds.
From www.pinterest.com
What is the difference between the ionic radius and the atomic radius Radius Ratio And Geometry Of Ionic Compounds This rule indicates that for an ionic compound to be stable, there is a critical range of radius ratios that can influence the coordination number, which. Importance of the radius ratio rule The structure of many ionic solids can be accounted by considering the relative sizes of the cation and anion, and their relative numbers. The table below gives the. Radius Ratio And Geometry Of Ionic Compounds.
From www.toppr.com
Radius Ratio Rule Ionic Model & Radius, Definition, Importance, Examples Radius Ratio And Geometry Of Ionic Compounds Hence, radius ration ρ = r s /r l. The structure of many ionic solids can be accounted by considering the relative sizes of the cation and anion, and their relative numbers. Radius ratio refers to as the ration of smaller ionic radius (cation) by the ratio of larger ionic radius (anion). Ionic model & ionic radius, definition of radius. Radius Ratio And Geometry Of Ionic Compounds.
From socratic.org
How does ionic radius change across a period? Socratic Radius Ratio And Geometry Of Ionic Compounds This rule indicates that for an ionic compound to be stable, there is a critical range of radius ratios that can influence the coordination number, which. The model of radius ratio allows a prediction of the coordination number (cn) in an ionic compound. Ionic model & ionic radius, definition of radius ratio, calculation of limiting radius ratio values, limitations, practice.. Radius Ratio And Geometry Of Ionic Compounds.
From www.youtube.com
8.2 Ceramics Ionic Radius Ratio, Crystal Structure, APF, and Radius Ratio And Geometry Of Ionic Compounds Radius ratio rules in ionic solids: The table below gives the ranges of cation/anion radius ratios that give the best fit for a given coordination geometry. Ionic model & ionic radius, definition of radius ratio, calculation of limiting radius ratio values, limitations, practice. The structure of many ionic solids can be accounted by considering the relative sizes of the cation. Radius Ratio And Geometry Of Ionic Compounds.
From glossary.periodni.com
Ionic Chemistry Dictionary & Glossary Radius Ratio And Geometry Of Ionic Compounds Hence, radius ration ρ = r s /r l. Importance of the radius ratio rule This rule indicates that for an ionic compound to be stable, there is a critical range of radius ratios that can influence the coordination number, which. Ionic model & ionic radius, definition of radius ratio, calculation of limiting radius ratio values, limitations, practice. The model. Radius Ratio And Geometry Of Ionic Compounds.
From www.slideshare.net
Lecture 02 Radius Ratio And Geometry Of Ionic Compounds This rule indicates that for an ionic compound to be stable, there is a critical range of radius ratios that can influence the coordination number, which. Radius ratio rules in ionic solids: Hence, radius ration ρ = r s /r l. The model of radius ratio allows a prediction of the coordination number (cn) in an ionic compound. Ionic model. Radius Ratio And Geometry Of Ionic Compounds.
From opengeology.org
13 Crystal Structures Mineralogy Radius Ratio And Geometry Of Ionic Compounds This rule indicates that for an ionic compound to be stable, there is a critical range of radius ratios that can influence the coordination number, which. Importance of the radius ratio rule The table below gives the ranges of cation/anion radius ratios that give the best fit for a given coordination geometry. The structure of many ionic solids can be. Radius Ratio And Geometry Of Ionic Compounds.
From www.youtube.com
Radius ratio ! Limitations of radius ratio ! Properties of ionic solid Radius Ratio And Geometry Of Ionic Compounds In order to derive the minimum. The table below gives the ranges of cation/anion radius ratios that give the best fit for a given coordination geometry. Radius ratio rule for ionic compounds •(1) in crystals, cations tend to get surrounded by the largest possible number of anions around it. This rule indicates that for an ionic compound to be stable,. Radius Ratio And Geometry Of Ionic Compounds.
From www.youtube.com
NEET/IITJEE chemistry,12th solid state,radius ratio rules for ionic Radius Ratio And Geometry Of Ionic Compounds Radius ratio rules in ionic solids: Ionic model & ionic radius, definition of radius ratio, calculation of limiting radius ratio values, limitations, practice. Hence, radius ration ρ = r s /r l. The structure of many ionic solids can be accounted by considering the relative sizes of the cation and anion, and their relative numbers. This rule indicates that for. Radius Ratio And Geometry Of Ionic Compounds.
From sciencenotes.org
Atomic Radius and Ionic Radius Radius Ratio And Geometry Of Ionic Compounds Ionic model & ionic radius, definition of radius ratio, calculation of limiting radius ratio values, limitations, practice. This rule indicates that for an ionic compound to be stable, there is a critical range of radius ratios that can influence the coordination number, which. The model of radius ratio allows a prediction of the coordination number (cn) in an ionic compound.. Radius Ratio And Geometry Of Ionic Compounds.
From www.youtube.com
How To Draw The Lewis Structures of Ionic Compounds YouTube Radius Ratio And Geometry Of Ionic Compounds Importance of the radius ratio rule This rule indicates that for an ionic compound to be stable, there is a critical range of radius ratios that can influence the coordination number, which. Radius ratio rule for ionic compounds •(1) in crystals, cations tend to get surrounded by the largest possible number of anions around it. The structure of many ionic. Radius Ratio And Geometry Of Ionic Compounds.
From askfilo.com
Radius ratio of an ionic compound is 0.93. The structure of the above io.. Radius Ratio And Geometry Of Ionic Compounds This rule indicates that for an ionic compound to be stable, there is a critical range of radius ratios that can influence the coordination number, which. The structure of many ionic solids can be accounted by considering the relative sizes of the cation and anion, and their relative numbers. Hence, radius ration ρ = r s /r l. The model. Radius Ratio And Geometry Of Ionic Compounds.
From www.youtube.com
Calculating the limiting radius ratio YouTube Radius Ratio And Geometry Of Ionic Compounds The structure of many ionic solids can be accounted by considering the relative sizes of the cation and anion, and their relative numbers. Radius ratio rules in ionic solids: Ionic model & ionic radius, definition of radius ratio, calculation of limiting radius ratio values, limitations, practice. The table below gives the ranges of cation/anion radius ratios that give the best. Radius Ratio And Geometry Of Ionic Compounds.
From chemistnotes.com
Radius Ratio The radius ratio rule Chemistry Notes Radius Ratio And Geometry Of Ionic Compounds The table below gives the ranges of cation/anion radius ratios that give the best fit for a given coordination geometry. Radius ratio refers to as the ration of smaller ionic radius (cation) by the ratio of larger ionic radius (anion). The structure of many ionic solids can be accounted by considering the relative sizes of the cation and anion, and. Radius Ratio And Geometry Of Ionic Compounds.
From askfilo.com
Radius ratio of an ionic compound is 0.93. The structure of the above io.. Radius Ratio And Geometry Of Ionic Compounds Radius ratio rule for ionic compounds •(1) in crystals, cations tend to get surrounded by the largest possible number of anions around it. Radius ratio refers to as the ration of smaller ionic radius (cation) by the ratio of larger ionic radius (anion). Ionic model & ionic radius, definition of radius ratio, calculation of limiting radius ratio values, limitations, practice.. Radius Ratio And Geometry Of Ionic Compounds.
From scientifictutor.org
Chem College Ionic Radius (Ionic Size) Scientific Tutor Radius Ratio And Geometry Of Ionic Compounds The structure of many ionic solids can be accounted by considering the relative sizes of the cation and anion, and their relative numbers. Hence, radius ration ρ = r s /r l. The table below gives the ranges of cation/anion radius ratios that give the best fit for a given coordination geometry. Radius ratio refers to as the ration of. Radius Ratio And Geometry Of Ionic Compounds.
From socratic.org
How does the ionic radius of a typical anion compare? Socratic Radius Ratio And Geometry Of Ionic Compounds Ionic model & ionic radius, definition of radius ratio, calculation of limiting radius ratio values, limitations, practice. Importance of the radius ratio rule Hence, radius ration ρ = r s /r l. Radius ratio refers to as the ration of smaller ionic radius (cation) by the ratio of larger ionic radius (anion). The table below gives the ranges of cation/anion. Radius Ratio And Geometry Of Ionic Compounds.
From www.pearson.com
In the ionic compounds LiF, NaCl, KBr, and RbI, the measured cat Radius Ratio And Geometry Of Ionic Compounds Radius ratio refers to as the ration of smaller ionic radius (cation) by the ratio of larger ionic radius (anion). Radius ratio rule for ionic compounds •(1) in crystals, cations tend to get surrounded by the largest possible number of anions around it. Radius ratio rules in ionic solids: Hence, radius ration ρ = r s /r l. This rule. Radius Ratio And Geometry Of Ionic Compounds.
From chem.libretexts.org
4.3 Formulas for Ionic Compounds Chemistry LibreTexts Radius Ratio And Geometry Of Ionic Compounds Hence, radius ration ρ = r s /r l. The structure of many ionic solids can be accounted by considering the relative sizes of the cation and anion, and their relative numbers. Ionic model & ionic radius, definition of radius ratio, calculation of limiting radius ratio values, limitations, practice. The model of radius ratio allows a prediction of the coordination. Radius Ratio And Geometry Of Ionic Compounds.
From slidetodoc.com
Radius ratio In ionic solids cations try to Radius Ratio And Geometry Of Ionic Compounds Ionic model & ionic radius, definition of radius ratio, calculation of limiting radius ratio values, limitations, practice. The model of radius ratio allows a prediction of the coordination number (cn) in an ionic compound. In order to derive the minimum. Radius ratio rule for ionic compounds •(1) in crystals, cations tend to get surrounded by the largest possible number of. Radius Ratio And Geometry Of Ionic Compounds.
From in.pinterest.com
Radius ratio. in 2021 Radii, Chemistry, Ionic Radius Ratio And Geometry Of Ionic Compounds Radius ratio rule for ionic compounds •(1) in crystals, cations tend to get surrounded by the largest possible number of anions around it. This rule indicates that for an ionic compound to be stable, there is a critical range of radius ratios that can influence the coordination number, which. The table below gives the ranges of cation/anion radius ratios that. Radius Ratio And Geometry Of Ionic Compounds.