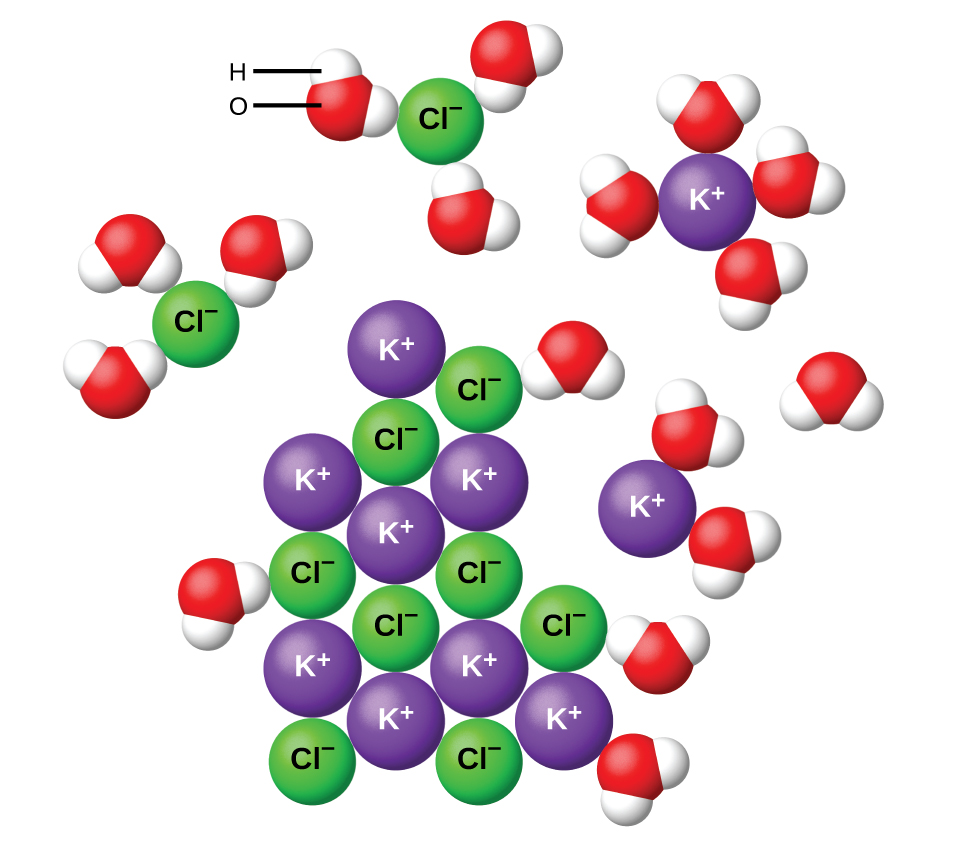
Which Molecules Dissolve In Water . Water, which not only dissolves many compounds but also dissolves more substances than any other. In fact, the heat of solution of. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. For example, nacl has a value of ∆ solh = +3 kj/mol, yet it dissolves in water. It is a polar molecule, allowing for the formation of hydrogen bonds. The tea ball holds the tea inside a mesh so that the leaves don’t escape, making your tea into a For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. The properties of liquids depend on the attractions the molecules of the liquid have for each other and for other substances. In tea, water is the solvent, and the molecules released by the tea leaves are the solutes (they dissolve in the water). Dipole forces and hydrogen bonding will tend to hold the water molecules together, but there are only weak london forces between water and nonpolar molecules. Hydrogen bonds allow ions and other polar molecules to dissolve in water.
from socratic.org
Hydrogen bonds allow ions and other polar molecules to dissolve in water. For example, nacl has a value of ∆ solh = +3 kj/mol, yet it dissolves in water. For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. Water, which not only dissolves many compounds but also dissolves more substances than any other. The properties of liquids depend on the attractions the molecules of the liquid have for each other and for other substances. Dipole forces and hydrogen bonding will tend to hold the water molecules together, but there are only weak london forces between water and nonpolar molecules. It is a polar molecule, allowing for the formation of hydrogen bonds. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. In fact, the heat of solution of. In tea, water is the solvent, and the molecules released by the tea leaves are the solutes (they dissolve in the water).
Question 0b448 Socratic
Which Molecules Dissolve In Water Dipole forces and hydrogen bonding will tend to hold the water molecules together, but there are only weak london forces between water and nonpolar molecules. Hydrogen bonds allow ions and other polar molecules to dissolve in water. In tea, water is the solvent, and the molecules released by the tea leaves are the solutes (they dissolve in the water). It is a polar molecule, allowing for the formation of hydrogen bonds. The tea ball holds the tea inside a mesh so that the leaves don’t escape, making your tea into a The properties of liquids depend on the attractions the molecules of the liquid have for each other and for other substances. In fact, the heat of solution of. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. For example, nacl has a value of ∆ solh = +3 kj/mol, yet it dissolves in water. For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. Dipole forces and hydrogen bonding will tend to hold the water molecules together, but there are only weak london forces between water and nonpolar molecules. Water, which not only dissolves many compounds but also dissolves more substances than any other.
From draw-hub.blogspot.com
Do Polar Or Nonpolar Molecules Dissolve In Water Drawhub Which Molecules Dissolve In Water The tea ball holds the tea inside a mesh so that the leaves don’t escape, making your tea into a For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. For example, nacl has a value of ∆ solh = +3 kj/mol, yet it dissolves in water. Water, which not. Which Molecules Dissolve In Water.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation ID1977818 Which Molecules Dissolve In Water Hydrogen bonds allow ions and other polar molecules to dissolve in water. The tea ball holds the tea inside a mesh so that the leaves don’t escape, making your tea into a In tea, water is the solvent, and the molecules released by the tea leaves are the solutes (they dissolve in the water). For instance, the following compounds, all. Which Molecules Dissolve In Water.
From www.slideserve.com
PPT Chapter 5 Molecular View of Reactions in Aqueous Solutions Part I Which Molecules Dissolve In Water The tea ball holds the tea inside a mesh so that the leaves don’t escape, making your tea into a In fact, the heat of solution of. Dipole forces and hydrogen bonding will tend to hold the water molecules together, but there are only weak london forces between water and nonpolar molecules. The properties of liquids depend on the attractions. Which Molecules Dissolve In Water.
From stock.adobe.com
How does sodium chloride (NaCl) dissolve in water Векторный объект Which Molecules Dissolve In Water Dipole forces and hydrogen bonding will tend to hold the water molecules together, but there are only weak london forces between water and nonpolar molecules. The properties of liquids depend on the attractions the molecules of the liquid have for each other and for other substances. For instance, the following compounds, all of which are polar and can form hydrogen. Which Molecules Dissolve In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation ID2276550 Which Molecules Dissolve In Water It is a polar molecule, allowing for the formation of hydrogen bonds. In tea, water is the solvent, and the molecules released by the tea leaves are the solutes (they dissolve in the water). Hydrogen bonds allow ions and other polar molecules to dissolve in water. When ionic compounds dissolve in water, the ions in the solid separate and disperse. Which Molecules Dissolve In Water.
From mybios.me
Chemical Makeup Of Salt Water My Bios Which Molecules Dissolve In Water For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. The tea ball holds the tea inside a mesh so that the leaves don’t escape, making your tea into a When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. Water,. Which Molecules Dissolve In Water.
From www.bbc.co.uk
Which materials dissolve in water? BBC Bitesize Which Molecules Dissolve In Water It is a polar molecule, allowing for the formation of hydrogen bonds. Hydrogen bonds allow ions and other polar molecules to dissolve in water. The properties of liquids depend on the attractions the molecules of the liquid have for each other and for other substances. Water, which not only dissolves many compounds but also dissolves more substances than any other.. Which Molecules Dissolve In Water.
From socratic.org
Question 0b448 Socratic Which Molecules Dissolve In Water In fact, the heat of solution of. The tea ball holds the tea inside a mesh so that the leaves don’t escape, making your tea into a The properties of liquids depend on the attractions the molecules of the liquid have for each other and for other substances. In tea, water is the solvent, and the molecules released by the. Which Molecules Dissolve In Water.
From www.numerade.com
SOLVED Describe how water molecules interact with sodium chloride Which Molecules Dissolve In Water Water, which not only dissolves many compounds but also dissolves more substances than any other. Dipole forces and hydrogen bonding will tend to hold the water molecules together, but there are only weak london forces between water and nonpolar molecules. For example, nacl has a value of ∆ solh = +3 kj/mol, yet it dissolves in water. The properties of. Which Molecules Dissolve In Water.
From www.slideserve.com
PPT Factors Affecting Solubility PowerPoint Presentation, free Which Molecules Dissolve In Water The tea ball holds the tea inside a mesh so that the leaves don’t escape, making your tea into a For example, nacl has a value of ∆ solh = +3 kj/mol, yet it dissolves in water. It is a polar molecule, allowing for the formation of hydrogen bonds. The properties of liquids depend on the attractions the molecules of. Which Molecules Dissolve In Water.
From www.chemistrysteps.com
Solubility of Organic Compounds Chemistry Steps Which Molecules Dissolve In Water Dipole forces and hydrogen bonding will tend to hold the water molecules together, but there are only weak london forces between water and nonpolar molecules. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. Hydrogen bonds allow ions and other polar molecules to dissolve in water. It is a polar. Which Molecules Dissolve In Water.
From www.expii.com
High Specific Heat (Water) — Properties & Examples Expii Which Molecules Dissolve In Water The properties of liquids depend on the attractions the molecules of the liquid have for each other and for other substances. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. In fact, the heat of solution of. The tea ball holds the tea inside a mesh so that the leaves. Which Molecules Dissolve In Water.
From www.sciencelearn.org.nz
Sugar dissolving in water — Science Learning Hub Which Molecules Dissolve In Water For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. The tea ball holds the tea inside a mesh so that the leaves don’t escape, making your tea into a In fact, the heat of solution of. When ionic compounds dissolve in water, the ions in the solid separate and. Which Molecules Dissolve In Water.
From socratic.org
What determines whether a solid is soluble in water? Socratic Which Molecules Dissolve In Water Dipole forces and hydrogen bonding will tend to hold the water molecules together, but there are only weak london forces between water and nonpolar molecules. For example, nacl has a value of ∆ solh = +3 kj/mol, yet it dissolves in water. In fact, the heat of solution of. It is a polar molecule, allowing for the formation of hydrogen. Which Molecules Dissolve In Water.
From sciencenotes.org
Why Is Water Called the Universal Solvent? Which Molecules Dissolve In Water In fact, the heat of solution of. Dipole forces and hydrogen bonding will tend to hold the water molecules together, but there are only weak london forces between water and nonpolar molecules. For example, nacl has a value of ∆ solh = +3 kj/mol, yet it dissolves in water. In tea, water is the solvent, and the molecules released by. Which Molecules Dissolve In Water.
From www.youtube.com
Solubility of O2 (Oxygen gas) in Water YouTube Which Molecules Dissolve In Water For example, nacl has a value of ∆ solh = +3 kj/mol, yet it dissolves in water. In fact, the heat of solution of. For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. The tea ball holds the tea inside a mesh so that the leaves don’t escape, making. Which Molecules Dissolve In Water.
From brainly.in
Draw a neat well labelled diagram of information of water molecule Which Molecules Dissolve In Water For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. The properties of liquids depend on the attractions the molecules of the liquid have for each other and for other substances. Hydrogen bonds allow ions and other polar molecules to dissolve in water. When ionic compounds dissolve in water, the. Which Molecules Dissolve In Water.
From www.slideserve.com
PPT Chemistry in Biology PowerPoint Presentation, free download ID Which Molecules Dissolve In Water It is a polar molecule, allowing for the formation of hydrogen bonds. Water, which not only dissolves many compounds but also dissolves more substances than any other. In fact, the heat of solution of. The properties of liquids depend on the attractions the molecules of the liquid have for each other and for other substances. When ionic compounds dissolve in. Which Molecules Dissolve In Water.
From pressbooks.bccampus.ca
6.3 AcidBase Reactions CHEM 1114 Introduction to Chemistry Which Molecules Dissolve In Water The properties of liquids depend on the attractions the molecules of the liquid have for each other and for other substances. It is a polar molecule, allowing for the formation of hydrogen bonds. Dipole forces and hydrogen bonding will tend to hold the water molecules together, but there are only weak london forces between water and nonpolar molecules. Water, which. Which Molecules Dissolve In Water.
From www.slideserve.com
PPT Solutes, Solutions and Solvents PowerPoint Presentation, free Which Molecules Dissolve In Water In tea, water is the solvent, and the molecules released by the tea leaves are the solutes (they dissolve in the water). The properties of liquids depend on the attractions the molecules of the liquid have for each other and for other substances. Dipole forces and hydrogen bonding will tend to hold the water molecules together, but there are only. Which Molecules Dissolve In Water.
From www.twinkl.fr
What is Dissolving? Answered Twinkl Teaching Wiki Which Molecules Dissolve In Water Hydrogen bonds allow ions and other polar molecules to dissolve in water. In fact, the heat of solution of. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. In tea, water is the solvent, and the molecules released by the tea leaves are the solutes (they dissolve in the water).. Which Molecules Dissolve In Water.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download Which Molecules Dissolve In Water For example, nacl has a value of ∆ solh = +3 kj/mol, yet it dissolves in water. It is a polar molecule, allowing for the formation of hydrogen bonds. Hydrogen bonds allow ions and other polar molecules to dissolve in water. In tea, water is the solvent, and the molecules released by the tea leaves are the solutes (they dissolve. Which Molecules Dissolve In Water.
From www.pinterest.com
Why Is Water a Polar Molecule? Water molecule, Molecular geometry Which Molecules Dissolve In Water In fact, the heat of solution of. The properties of liquids depend on the attractions the molecules of the liquid have for each other and for other substances. For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. Hydrogen bonds allow ions and other polar molecules to dissolve in water.. Which Molecules Dissolve In Water.
From www.gauthmath.com
Solved Which types of molecules dissolve in water Time 115 polar Which Molecules Dissolve In Water In fact, the heat of solution of. Dipole forces and hydrogen bonding will tend to hold the water molecules together, but there are only weak london forces between water and nonpolar molecules. For example, nacl has a value of ∆ solh = +3 kj/mol, yet it dissolves in water. The properties of liquids depend on the attractions the molecules of. Which Molecules Dissolve In Water.
From www.science-sparks.com
Which solids dissolve in water Which Molecules Dissolve In Water The tea ball holds the tea inside a mesh so that the leaves don’t escape, making your tea into a When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. Hydrogen bonds allow ions and other polar molecules to dissolve in water. In fact, the heat of solution of. Dipole forces. Which Molecules Dissolve In Water.
From mungfali.com
Dissolving Salt In Water Diagram Which Molecules Dissolve In Water Water, which not only dissolves many compounds but also dissolves more substances than any other. For example, nacl has a value of ∆ solh = +3 kj/mol, yet it dissolves in water. Dipole forces and hydrogen bonding will tend to hold the water molecules together, but there are only weak london forces between water and nonpolar molecules. When ionic compounds. Which Molecules Dissolve In Water.
From www.baristahustle.com
TWC 0.02 How Does Water Dissolve Mineral Salts? Barista Hustle Which Molecules Dissolve In Water Hydrogen bonds allow ions and other polar molecules to dissolve in water. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. Dipole forces and hydrogen bonding will tend to hold the water molecules together, but there are only weak london forces between water and nonpolar molecules. For instance, the following. Which Molecules Dissolve In Water.
From www.slideserve.com
PPT Unit 6 TOXINS Solutions & PowerPoint Presentation ID Which Molecules Dissolve In Water In fact, the heat of solution of. Dipole forces and hydrogen bonding will tend to hold the water molecules together, but there are only weak london forces between water and nonpolar molecules. The tea ball holds the tea inside a mesh so that the leaves don’t escape, making your tea into a For instance, the following compounds, all of which. Which Molecules Dissolve In Water.
From www.expii.com
Good Solvent (Water) — Properties & Examples Expii Which Molecules Dissolve In Water In tea, water is the solvent, and the molecules released by the tea leaves are the solutes (they dissolve in the water). When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. The tea ball holds the tea inside a mesh so that the leaves don’t escape, making your tea into. Which Molecules Dissolve In Water.
From www.scienceabc.com
Why Does Sugar Disappear When It Dissolves In Water? » ScienceABC Which Molecules Dissolve In Water Dipole forces and hydrogen bonding will tend to hold the water molecules together, but there are only weak london forces between water and nonpolar molecules. The tea ball holds the tea inside a mesh so that the leaves don’t escape, making your tea into a For instance, the following compounds, all of which are polar and can form hydrogen bonds. Which Molecules Dissolve In Water.
From brainly.in
The image shows particles of salt dissolved in water. Brainly.in Which Molecules Dissolve In Water The properties of liquids depend on the attractions the molecules of the liquid have for each other and for other substances. For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. In fact, the heat of solution of. Dipole forces and hydrogen bonding will tend to hold the water molecules. Which Molecules Dissolve In Water.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Which Molecules Dissolve In Water Hydrogen bonds allow ions and other polar molecules to dissolve in water. Water, which not only dissolves many compounds but also dissolves more substances than any other. In tea, water is the solvent, and the molecules released by the tea leaves are the solutes (they dissolve in the water). It is a polar molecule, allowing for the formation of hydrogen. Which Molecules Dissolve In Water.
From www.researchgate.net
Schematic diagram showing interaction of water molecules with Which Molecules Dissolve In Water For example, nacl has a value of ∆ solh = +3 kj/mol, yet it dissolves in water. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. The tea ball holds the tea inside a mesh so that the leaves don’t escape, making your tea into a The properties of liquids. Which Molecules Dissolve In Water.
From slideplayer.com
Energy Matters Compounds and Bonding ppt download Which Molecules Dissolve In Water Water, which not only dissolves many compounds but also dissolves more substances than any other. For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. It is a polar molecule, allowing for the formation of hydrogen bonds. The properties of liquids depend on the attractions the molecules of the liquid. Which Molecules Dissolve In Water.
From www.slideserve.com
PPT The Nature of Molecules and the Properties of Water PowerPoint Which Molecules Dissolve In Water Dipole forces and hydrogen bonding will tend to hold the water molecules together, but there are only weak london forces between water and nonpolar molecules. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. It is a polar molecule, allowing for the formation of hydrogen bonds. For example, nacl has. Which Molecules Dissolve In Water.