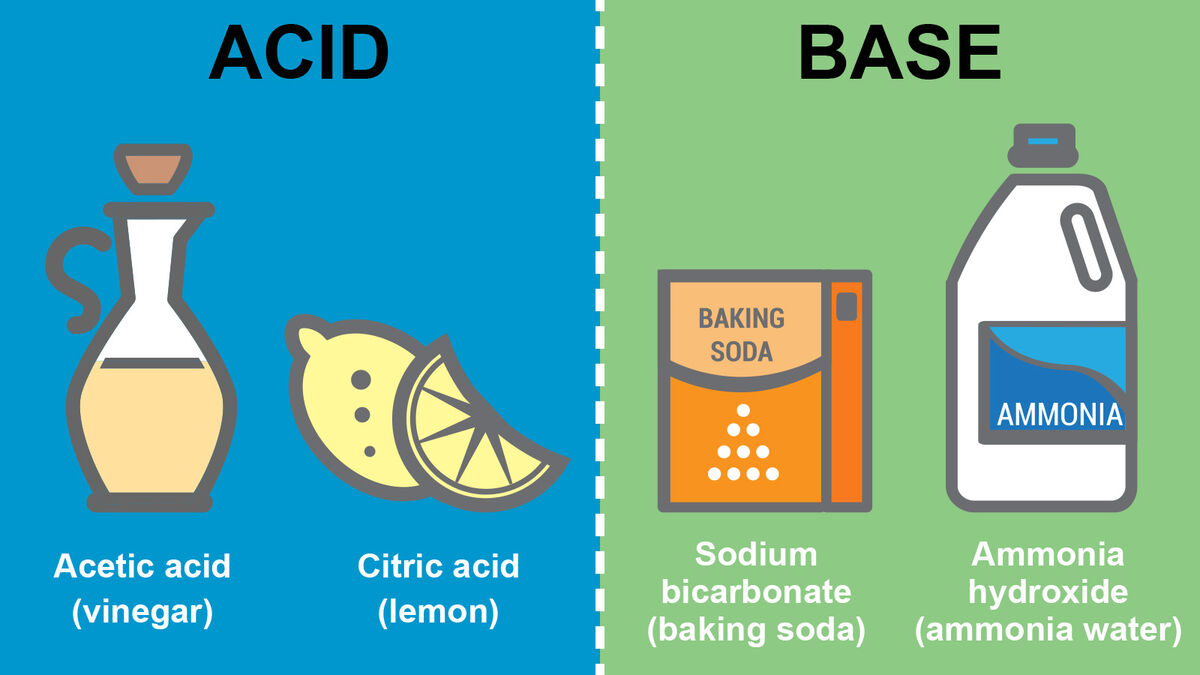
Example Of Concentrated Base . If a base is diluted, it contains a high amount of water. A solid base acts as a base in solid form. The acid and base share an electron pair. Concentrated bases have high percentages of base substances compared to their water levels. In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves in water. Sodium hydroxide is found in drain cleaner. Antacids, which combat excess stomach acid, are comprised of bases such as magnesium hydroxide or sodium. A simple example of a superbase is sodium hydride (nah). Types of bases based on their concentration in aqueous solution. The basic solution that contains a higher percentage of the base compound is known as concentrated base. Many cleaners contain ammonia, a base. A neutral base forms a bond with a neutral acid. If a base is diluted, it consists of an excessive quantity of water.
from klagiicuo.blob.core.windows.net
Types of bases based on their concentration in aqueous solution. Sodium hydroxide is found in drain cleaner. Concentrated bases have high percentages of base substances compared to their water levels. A neutral base forms a bond with a neutral acid. A simple example of a superbase is sodium hydride (nah). Many cleaners contain ammonia, a base. The basic solution that contains a higher percentage of the base compound is known as concentrated base. In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. Antacids, which combat excess stomach acid, are comprised of bases such as magnesium hydroxide or sodium. A solid base acts as a base in solid form.
Base Definition Chemistry Igcse at Carolyn Wright blog
Example Of Concentrated Base Concentrated bases have high percentages of base substances compared to their water levels. Types of bases based on their concentration in aqueous solution. A neutral base forms a bond with a neutral acid. Sodium hydroxide is found in drain cleaner. A simple example of a superbase is sodium hydride (nah). In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. The basic solution that contains a higher percentage of the base compound is known as concentrated base. The acid and base share an electron pair. Concentrated bases have high percentages of base substances compared to their water levels. If a base is diluted, it consists of an excessive quantity of water. If a base is diluted, it contains a high amount of water. A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves in water. Many cleaners contain ammonia, a base. A solid base acts as a base in solid form. Antacids, which combat excess stomach acid, are comprised of bases such as magnesium hydroxide or sodium.
From chem.libretexts.org
4.5 Concentration of Solutions Chemistry LibreTexts Example Of Concentrated Base Sodium hydroxide is found in drain cleaner. A solid base acts as a base in solid form. A neutral base forms a bond with a neutral acid. In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. Concentrated bases have high percentages of base substances compared to their water levels.. Example Of Concentrated Base.
From klagiicuo.blob.core.windows.net
Base Definition Chemistry Igcse at Carolyn Wright blog Example Of Concentrated Base A solid base acts as a base in solid form. A neutral base forms a bond with a neutral acid. In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. Many cleaners contain ammonia, a base. Types of bases based on their concentration in aqueous solution. Concentrated bases have high. Example Of Concentrated Base.
From www.slideserve.com
PPT Section 6.3—Acidity, pH PowerPoint Presentation, free download Example Of Concentrated Base Many cleaners contain ammonia, a base. If a base is diluted, it consists of an excessive quantity of water. If a base is diluted, it contains a high amount of water. In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. The acid and base share an electron pair. A. Example Of Concentrated Base.
From www.geeksforgeeks.org
Concentration of Solution Definition, Formulas & Solved Examples Example Of Concentrated Base The acid and base share an electron pair. If a base is diluted, it contains a high amount of water. If a base is diluted, it consists of an excessive quantity of water. Concentrated bases have high percentages of base substances compared to their water levels. A simple example of a superbase is sodium hydride (nah). A strong base, unlike. Example Of Concentrated Base.
From www.pinterest.com
Concentration Easy Science Physics concepts, Concentration, Medical Example Of Concentrated Base A simple example of a superbase is sodium hydride (nah). Antacids, which combat excess stomach acid, are comprised of bases such as magnesium hydroxide or sodium. A neutral base forms a bond with a neutral acid. A solid base acts as a base in solid form. Sodium hydroxide is found in drain cleaner. The basic solution that contains a higher. Example Of Concentrated Base.
From www.teachoo.com
Bases and it's Properties (with Examples, Definition) Teachoo Example Of Concentrated Base Antacids, which combat excess stomach acid, are comprised of bases such as magnesium hydroxide or sodium. If a base is diluted, it consists of an excessive quantity of water. If a base is diluted, it contains a high amount of water. A neutral base forms a bond with a neutral acid. Sodium hydroxide is found in drain cleaner. A solid. Example Of Concentrated Base.
From innobator.blogspot.com
INNOBATOR FÍSICA Y QUÍMICA 20212022 PHYSICS AND CHEMISTRY 2º ESO Example Of Concentrated Base Many cleaners contain ammonia, a base. If a base is diluted, it contains a high amount of water. The basic solution that contains a higher percentage of the base compound is known as concentrated base. A neutral base forms a bond with a neutral acid. A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves. Example Of Concentrated Base.
From www.slideserve.com
PPT Solutions Part II PowerPoint Presentation, free download ID4272422 Example Of Concentrated Base If a base is diluted, it consists of an excessive quantity of water. A simple example of a superbase is sodium hydride (nah). In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. A neutral base forms a bond with a neutral acid. A solid base acts as a base. Example Of Concentrated Base.
From www.conquerhsc.com
HSC Chemistry Module 6 Inquiry Question 2 Example Of Concentrated Base The acid and base share an electron pair. Types of bases based on their concentration in aqueous solution. If a base is diluted, it consists of an excessive quantity of water. Antacids, which combat excess stomach acid, are comprised of bases such as magnesium hydroxide or sodium. A solid base acts as a base in solid form. If a base. Example Of Concentrated Base.
From www.slideserve.com
PPT Water and the aqueous systems PowerPoint Presentation, free Example Of Concentrated Base A neutral base forms a bond with a neutral acid. Many cleaners contain ammonia, a base. In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. Types of bases based on their concentration in aqueous solution. The basic solution that contains a higher percentage of the base compound is known. Example Of Concentrated Base.
From www.teachoo.com
Classification of Acids on Basis of source, Concentration Teachoo Example Of Concentrated Base If a base is diluted, it consists of an excessive quantity of water. Sodium hydroxide is found in drain cleaner. The acid and base share an electron pair. In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. A solid base acts as a base in solid form. Types of. Example Of Concentrated Base.
From www.orringtonfarms.com
Premium Broth & Soup Base Concentrated Bases, Gravies, Meal Creations Example Of Concentrated Base A solid base acts as a base in solid form. A simple example of a superbase is sodium hydride (nah). In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. Types of bases based on their concentration in aqueous solution. The acid and base share an electron pair. If a. Example Of Concentrated Base.
From exovgpnzm.blob.core.windows.net
Dilution And Concentration Of Liquid at Nicole Gardner blog Example Of Concentrated Base The basic solution that contains a higher percentage of the base compound is known as concentrated base. The acid and base share an electron pair. Antacids, which combat excess stomach acid, are comprised of bases such as magnesium hydroxide or sodium. In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of. Example Of Concentrated Base.
From www.youtube.com
GCSE CHEMISTRY ACIDS AND BASES LESSON 3 base and alkali Example Of Concentrated Base Sodium hydroxide is found in drain cleaner. Many cleaners contain ammonia, a base. A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves in water. If a base is diluted, it contains a high amount of water. If a base is diluted, it consists of an excessive quantity of water. A solid base acts as. Example Of Concentrated Base.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration Example Of Concentrated Base Concentrated bases have high percentages of base substances compared to their water levels. The basic solution that contains a higher percentage of the base compound is known as concentrated base. In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. If a base is diluted, it consists of an excessive. Example Of Concentrated Base.
From threadreaderapp.com
Thread by Doc_MD18 "NMAT Review Must Know Chemistry [THREAD] (Topics Example Of Concentrated Base A solid base acts as a base in solid form. Many cleaners contain ammonia, a base. Concentrated bases have high percentages of base substances compared to their water levels. The basic solution that contains a higher percentage of the base compound is known as concentrated base. In the world of chemistry, bases are substances that can accept protons (h +. Example Of Concentrated Base.
From www.scribd.com
Examples+of+concentrated+solutions PDF Example Of Concentrated Base A neutral base forms a bond with a neutral acid. Sodium hydroxide is found in drain cleaner. Types of bases based on their concentration in aqueous solution. A simple example of a superbase is sodium hydride (nah). If a base is diluted, it contains a high amount of water. If a base is diluted, it consists of an excessive quantity. Example Of Concentrated Base.
From scienceready.com.au
Strength & Concentration of Acids/Bases HSC Chemistry Science Ready Example Of Concentrated Base The acid and base share an electron pair. Types of bases based on their concentration in aqueous solution. A solid base acts as a base in solid form. Concentrated bases have high percentages of base substances compared to their water levels. If a base is diluted, it consists of an excessive quantity of water. In the world of chemistry, bases. Example Of Concentrated Base.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Example Of Concentrated Base Antacids, which combat excess stomach acid, are comprised of bases such as magnesium hydroxide or sodium. If a base is diluted, it consists of an excessive quantity of water. A simple example of a superbase is sodium hydride (nah). A neutral base forms a bond with a neutral acid. A strong base, unlike a weak base, dissociates (separates) completely into. Example Of Concentrated Base.
From www.youtube.com
STRENGTH OF ACIDS AND BASES YouTube Example Of Concentrated Base In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. The acid and base share an electron pair. A solid base acts as a base in solid form. If a base is diluted, it consists of an excessive quantity of water. A neutral base forms a bond with a neutral. Example Of Concentrated Base.
From www.slideserve.com
PPT Solutions and Concentration (p123 136 ) PowerPoint Presentation Example Of Concentrated Base Many cleaners contain ammonia, a base. A neutral base forms a bond with a neutral acid. A simple example of a superbase is sodium hydride (nah). Concentrated bases have high percentages of base substances compared to their water levels. If a base is diluted, it contains a high amount of water. A solid base acts as a base in solid. Example Of Concentrated Base.
From www.youtube.com
Mixing Strong Concentrated Acid and Base YouTube Example Of Concentrated Base Antacids, which combat excess stomach acid, are comprised of bases such as magnesium hydroxide or sodium. Types of bases based on their concentration in aqueous solution. The basic solution that contains a higher percentage of the base compound is known as concentrated base. A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves in water.. Example Of Concentrated Base.
From www.teachoo.com
Classification of Acids on Basis of source, Concentration Teachoo Example Of Concentrated Base Types of bases based on their concentration in aqueous solution. A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves in water. A simple example of a superbase is sodium hydride (nah). Concentrated bases have high percentages of base substances compared to their water levels. Sodium hydroxide is found in drain cleaner. If a base. Example Of Concentrated Base.
From www.youtube.com
Aqueous, Dilute And Concentrated Solution, General Science Lecture Example Of Concentrated Base A simple example of a superbase is sodium hydride (nah). A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves in water. A neutral base forms a bond with a neutral acid. The basic solution that contains a higher percentage of the base compound is known as concentrated base. If a base is diluted, it. Example Of Concentrated Base.
From www.youtube.com
Concentrated acid or dilute acid acid base and salts10th class Example Of Concentrated Base If a base is diluted, it contains a high amount of water. Sodium hydroxide is found in drain cleaner. A solid base acts as a base in solid form. A simple example of a superbase is sodium hydride (nah). If a base is diluted, it consists of an excessive quantity of water. Antacids, which combat excess stomach acid, are comprised. Example Of Concentrated Base.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Example Of Concentrated Base Sodium hydroxide is found in drain cleaner. A neutral base forms a bond with a neutral acid. A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves in water. If a base is diluted, it consists of an excessive quantity of water. A solid base acts as a base in solid form. The basic solution. Example Of Concentrated Base.
From studylib.net
Acids and Bases Example Of Concentrated Base A neutral base forms a bond with a neutral acid. A solid base acts as a base in solid form. If a base is diluted, it contains a high amount of water. Sodium hydroxide is found in drain cleaner. Many cleaners contain ammonia, a base. In the world of chemistry, bases are substances that can accept protons (h + ions). Example Of Concentrated Base.
From www.exampleslab.com
20 Examples of Chemical Bases Examples Lab Example Of Concentrated Base Antacids, which combat excess stomach acid, are comprised of bases such as magnesium hydroxide or sodium. Types of bases based on their concentration in aqueous solution. Sodium hydroxide is found in drain cleaner. Many cleaners contain ammonia, a base. If a base is diluted, it consists of an excessive quantity of water. If a base is diluted, it contains a. Example Of Concentrated Base.
From chem.libretexts.org
4.3 Solution Concentrations Chemistry LibreTexts Example Of Concentrated Base Sodium hydroxide is found in drain cleaner. A neutral base forms a bond with a neutral acid. Many cleaners contain ammonia, a base. In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. Concentrated bases have high percentages of base substances compared to their water levels. The basic solution that. Example Of Concentrated Base.
From www.yaclass.in
Types of solutions Based on the amount of the solute — lesson. Science Example Of Concentrated Base Types of bases based on their concentration in aqueous solution. The acid and base share an electron pair. A solid base acts as a base in solid form. In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. Sodium hydroxide is found in drain cleaner. If a base is diluted,. Example Of Concentrated Base.
From minichemist.com
Mini HCBC™ Concentrated Base MiniChemist Store Example Of Concentrated Base If a base is diluted, it contains a high amount of water. The basic solution that contains a higher percentage of the base compound is known as concentrated base. Sodium hydroxide is found in drain cleaner. Antacids, which combat excess stomach acid, are comprised of bases such as magnesium hydroxide or sodium. A simple example of a superbase is sodium. Example Of Concentrated Base.
From www.conquerhsc.com
HSC Chemistry Module 6 Inquiry Question 2 Example Of Concentrated Base A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves in water. The acid and base share an electron pair. Concentrated bases have high percentages of base substances compared to their water levels. A solid base acts as a base in solid form. A simple example of a superbase is sodium hydride (nah). If a. Example Of Concentrated Base.
From www.youtube.com
Concentrated Acid vs. Strong Acid (Diagram and Explanations) YouTube Example Of Concentrated Base Types of bases based on their concentration in aqueous solution. The acid and base share an electron pair. In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves in water. If a base is diluted,. Example Of Concentrated Base.
From www.youtube.com
Finding concentration of acid or base given amount needed to titrate Example Of Concentrated Base A solid base acts as a base in solid form. The acid and base share an electron pair. If a base is diluted, it contains a high amount of water. A neutral base forms a bond with a neutral acid. The basic solution that contains a higher percentage of the base compound is known as concentrated base. A simple example. Example Of Concentrated Base.
From www.slideshare.net
Chemistry Chp 16 Solutions PowerPoint (shortened) Example Of Concentrated Base A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves in water. Sodium hydroxide is found in drain cleaner. A solid base acts as a base in solid form. The basic solution that contains a higher percentage of the base compound is known as concentrated base. A simple example of a superbase is sodium hydride. Example Of Concentrated Base.