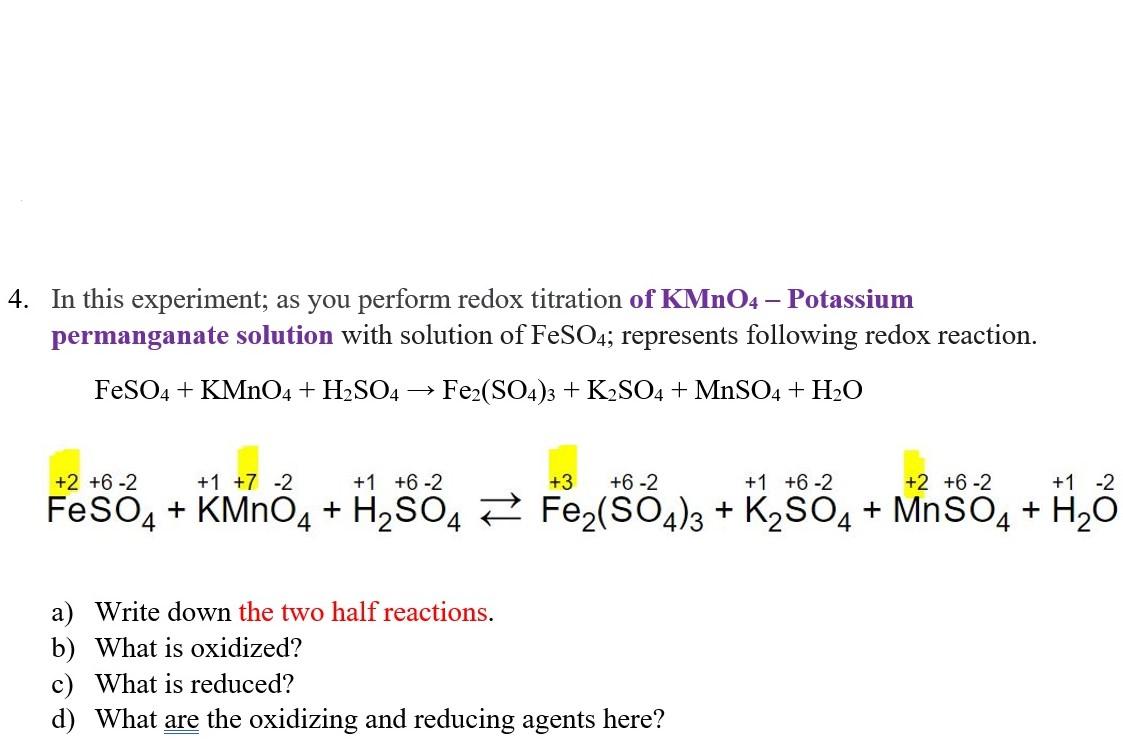
Titration Of Fe2+ With Kmno4 Reaction Equation . It is specifically mentioned in the specification but it could be any redox reaction. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. Write the balanced equation for the reaction oxidation: The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. Permanganate ion reduces to a. In the redox titration between potassium manganate (vii) and fe²⁺ ions the following reactions occur: The ionic equation involved in the process is given below. Fe³⁺ +e⁻ ⇌ fe²⁺ e⦵ = +0.77v. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution.
from xaydungso.vn
In the redox titration between potassium manganate (vii) and fe²⁺ ions the following reactions occur: Write the balanced equation for the reaction oxidation: Permanganate ion reduces to a. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. The ionic equation involved in the process is given below. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Fe³⁺ +e⁻ ⇌ fe²⁺ e⦵ = +0.77v. It is specifically mentioned in the specification but it could be any redox reaction. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,.
FeSO4 + KMnO4 + H2SO4 Phản ứng Hóa học và Ứng dụng Thực tiễn
Titration Of Fe2+ With Kmno4 Reaction Equation Fe³⁺ +e⁻ ⇌ fe²⁺ e⦵ = +0.77v. Permanganate ion reduces to a. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. In the redox titration between potassium manganate (vii) and fe²⁺ ions the following reactions occur: The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. It is specifically mentioned in the specification but it could be any redox reaction. Write the balanced equation for the reaction oxidation: The ionic equation involved in the process is given below. Fe³⁺ +e⁻ ⇌ fe²⁺ e⦵ = +0.77v. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,.
From www.numerade.com
SOLVED In the titration of ammonium iron (II) sulfate hexahydrate with Titration Of Fe2+ With Kmno4 Reaction Equation It is specifically mentioned in the specification but it could be any redox reaction. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. The ionic equation involved in the process is given below. Fe³⁺ +e⁻ ⇌ fe²⁺ e⦵ = +0.77v. In this experiment you will use a standard. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID2952040 Titration Of Fe2+ With Kmno4 Reaction Equation The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. Write the balanced equation for the reaction oxidation: It is specifically mentioned in the specification but it could be any redox reaction. In the redox titration between potassium manganate (vii) and fe²⁺ ions the following reactions occur: Permanganate ion. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.scribd.com
4 Estimation of Fe2 by KMnO4 PDF Titration Chemistry Titration Of Fe2+ With Kmno4 Reaction Equation In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Write the balanced equation for the reaction oxidation: The ionic equation involved in the process is given below. It is specifically mentioned in the specification but it could be any redox reaction. Permanganate ion. Titration Of Fe2+ With Kmno4 Reaction Equation.
From joizlqfat.blob.core.windows.net
Kmno4 Fe2+ Titration Equation at Linda Steel blog Titration Of Fe2+ With Kmno4 Reaction Equation Permanganate ion reduces to a. Write the balanced equation for the reaction oxidation: In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores.. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.youtube.com
Titration KMnO4 Vs Mohr Salt in Hindi Full Experiment Titration Of Fe2+ With Kmno4 Reaction Equation It is specifically mentioned in the specification but it could be any redox reaction. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. Write the balanced equation for the reaction oxidation: In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.youtube.com
Chemistry Redox Reaction Iron (II) (Fe2+) ion with permanganate ion Titration Of Fe2+ With Kmno4 Reaction Equation In the redox titration between potassium manganate (vii) and fe²⁺ ions the following reactions occur: Write the balanced equation for the reaction oxidation: It is specifically mentioned in the specification but it could be any redox reaction. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.youtube.com
Standardization of KMnO4 Redox Titration KMnO4 Vs Fe2+ Mohrs salt Titration Of Fe2+ With Kmno4 Reaction Equation The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. In the redox titration between potassium manganate (vii) and fe²⁺ ions the following reactions occur: Write the balanced equation for the reaction oxidation: It is specifically mentioned in the specification but it could be any redox reaction. Fe³⁺ +e⁻. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.youtube.com
titration of kmno4 with mohr's salt class 12 determine the Titration Of Fe2+ With Kmno4 Reaction Equation In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. The ionic equation involved in the process is given below. In the redox titration between potassium manganate (vii) and fe²⁺ ions the following reactions occur: The redox titration of permanganate ions is commonly used. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation, free download ID431911 Titration Of Fe2+ With Kmno4 Reaction Equation Fe³⁺ +e⁻ ⇌ fe²⁺ e⦵ = +0.77v. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. The ionic equation involved in the process is given below. It is specifically mentioned in the specification but it could be any redox reaction. In this experiment you will use a standard. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.numerade.com
SOLVED Experiment 12 OxidationReduction Reactions Potassium Titration Of Fe2+ With Kmno4 Reaction Equation Fe³⁺ +e⁻ ⇌ fe²⁺ e⦵ = +0.77v. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. In the redox titration between potassium manganate (vii) and fe²⁺ ions the following reactions occur: The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,.. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.studypool.com
SOLUTION Determination of Fe+² with kmno4 Reaction. Studypool Titration Of Fe2+ With Kmno4 Reaction Equation Permanganate ion reduces to a. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Fe³⁺ +e⁻ ⇌ fe²⁺ e⦵ = +0.77v. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. Write the balanced equation for. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.chegg.com
3.) For the following titration titration of Fe2+ Titration Of Fe2+ With Kmno4 Reaction Equation The ionic equation involved in the process is given below. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. In the redox titration between potassium manganate (vii) and fe²⁺ ions the following reactions occur: Fe³⁺ +e⁻ ⇌ fe²⁺ e⦵ = +0.77v. The redox. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.numerade.com
SOLVED86. So.00mL sample of solution containing Fe2t ions is titrated Titration Of Fe2+ With Kmno4 Reaction Equation Permanganate ion reduces to a. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. It is specifically mentioned in the specification but it could be any redox reaction. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.toppr.com
KMnO4 acts as an oxidizing agent in acidic medium. The number of moles Titration Of Fe2+ With Kmno4 Reaction Equation The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. In the redox titration between potassium manganate (vii) and fe²⁺ ions the following reactions occur: The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. The ionic equation involved in the process. Titration Of Fe2+ With Kmno4 Reaction Equation.
From xaydungso.vn
FeSO4 + KMnO4 + H2SO4 Phản ứng Hóa học và Ứng dụng Thực tiễn Titration Of Fe2+ With Kmno4 Reaction Equation The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. The ionic equation involved in the process is given below. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. It is specifically mentioned in the specification but it could be any. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.coursehero.com
[Solved] What is the balanced equation between KMnO4 and FeCl2. (For Titration Of Fe2+ With Kmno4 Reaction Equation Permanganate ion reduces to a. It is specifically mentioned in the specification but it could be any redox reaction. The ionic equation involved in the process is given below. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. In the redox titration between potassium manganate (vii) and fe²⁺ ions the following reactions. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.youtube.com
General Chemistry Standardization of KMnO4 By Redox Titration YouTube Titration Of Fe2+ With Kmno4 Reaction Equation It is specifically mentioned in the specification but it could be any redox reaction. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. The ionic equation involved in the process is given below. In the redox titration between potassium manganate (vii) and fe²⁺ ions the following reactions occur: Fe³⁺ +e⁻ ⇌ fe²⁺. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.chegg.com
Solved Sodium oxalate can be titrated with KMnO4 under Titration Of Fe2+ With Kmno4 Reaction Equation Fe³⁺ +e⁻ ⇌ fe²⁺ e⦵ = +0.77v. In the redox titration between potassium manganate (vii) and fe²⁺ ions the following reactions occur: In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. The redox titration of permanganate ions is commonly used to determine the. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.youtube.com
How to Balance KMnO4 + Fe = FeO + K2O + MnO2 YouTube Titration Of Fe2+ With Kmno4 Reaction Equation In the redox titration between potassium manganate (vii) and fe²⁺ ions the following reactions occur: It is specifically mentioned in the specification but it could be any redox reaction. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. In this experiment you will use a standard solution of potassium permanganate (kmno 4). Titration Of Fe2+ With Kmno4 Reaction Equation.
From slideplayer.com
Lesson 6 Redox Titrations ppt download Titration Of Fe2+ With Kmno4 Reaction Equation It is specifically mentioned in the specification but it could be any redox reaction. The ionic equation involved in the process is given below. Write the balanced equation for the reaction oxidation: Fe³⁺ +e⁻ ⇌ fe²⁺ e⦵ = +0.77v. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. In the redox titration. Titration Of Fe2+ With Kmno4 Reaction Equation.
From byjus.com
Mohr Salt Titration with KMnO4 CBSE Chemistry Practicals Class 12 Titration Of Fe2+ With Kmno4 Reaction Equation In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. In the redox titration between potassium manganate (vii) and fe²⁺ ions the following reactions occur: Fe³⁺ +e⁻ ⇌ fe²⁺ e⦵ = +0.77v. Write the balanced equation for the reaction oxidation: It is specifically mentioned. Titration Of Fe2+ With Kmno4 Reaction Equation.
From slideplayer.com
Balance the following MnO4 Mn2+ Fe2+ Fe ppt download Titration Of Fe2+ With Kmno4 Reaction Equation The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. In the redox titration between potassium manganate (vii) and fe²⁺ ions the following reactions occur: In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.numerade.com
SOLVED A standardized 0.153 mol/L solution of KMnO4(aq) was used to Titration Of Fe2+ With Kmno4 Reaction Equation It is specifically mentioned in the specification but it could be any redox reaction. In the redox titration between potassium manganate (vii) and fe²⁺ ions the following reactions occur: The ionic equation involved in the process is given below. Fe³⁺ +e⁻ ⇌ fe²⁺ e⦵ = +0.77v. In this experiment you will use a standard solution of potassium permanganate (kmno 4). Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.numerade.com
SOLVED The iron content of drinking water can be measured by titration Titration Of Fe2+ With Kmno4 Reaction Equation It is specifically mentioned in the specification but it could be any redox reaction. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. Permanganate ion reduces. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.numerade.com
SOLVED In the titration of ammonium iron (II) sulfate hexahydrate with Titration Of Fe2+ With Kmno4 Reaction Equation Fe³⁺ +e⁻ ⇌ fe²⁺ e⦵ = +0.77v. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. In the redox titration between potassium manganate (vii) and fe²⁺ ions the following reactions occur: The redox titration of permanganate ions is commonly used to determine the. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.youtube.com
Titration KMnO4 Vs Mohr's Salt Full Experiment + Calculation YouTube Titration Of Fe2+ With Kmno4 Reaction Equation Fe³⁺ +e⁻ ⇌ fe²⁺ e⦵ = +0.77v. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. Permanganate ion reduces to a. Write the balanced equation for the reaction oxidation: The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. It is. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.youtube.com
Redox Titration between MnO4 and Fe2+ YouTube Titration Of Fe2+ With Kmno4 Reaction Equation The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. The ionic equation involved in the process is given below. Write the balanced equation for the reaction oxidation: Permanganate ion reduces to a. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.congress-intercultural.eu
To Determine The Molarity Of KMnO4 Solution By Titrating It, 44 OFF Titration Of Fe2+ With Kmno4 Reaction Equation The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. Write the balanced equation for the reaction oxidation: It is specifically mentioned in the specification but it could be any redox reaction. In the redox titration between potassium manganate (vii) and fe²⁺ ions the following reactions occur: Permanganate ion reduces to a. The. Titration Of Fe2+ With Kmno4 Reaction Equation.
From joizlqfat.blob.core.windows.net
Kmno4 Fe2+ Titration Equation at Linda Steel blog Titration Of Fe2+ With Kmno4 Reaction Equation In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. In the redox titration between potassium manganate (vii) and fe²⁺ ions the following reactions occur: The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. Permanganate ion. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.youtube.com
Titration Of Kmno4 Vs Oxalic Acid The Ultimate Guide Titration Titration Of Fe2+ With Kmno4 Reaction Equation The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. In the redox titration between potassium manganate (vii) and fe²⁺ ions the following reactions occur: Write the balanced equation for the reaction. Titration Of Fe2+ With Kmno4 Reaction Equation.
From joizlqfat.blob.core.windows.net
Kmno4 Fe2+ Titration Equation at Linda Steel blog Titration Of Fe2+ With Kmno4 Reaction Equation In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Permanganate ion reduces to a. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. The ionic equation involved in the process is given below. Write the. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.numerade.com
SOLVED Write the Redox titration report for determination of Fe2 Titration Of Fe2+ With Kmno4 Reaction Equation Write the balanced equation for the reaction oxidation: Fe³⁺ +e⁻ ⇌ fe²⁺ e⦵ = +0.77v. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Permanganate ion reduces to a. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.numerade.com
SOLVED Part I Standardization of the KMnO4 solution Show the two half Titration Of Fe2+ With Kmno4 Reaction Equation The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. Permanganate ion reduces to a. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Fe³⁺ +e⁻ ⇌ fe²⁺ e⦵ = +0.77v. The redox titration of permanganate. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.chegg.com
Solved Experiment No. 06 ExperimentName Standardization of Titration Of Fe2+ With Kmno4 Reaction Equation The ionic equation involved in the process is given below. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Write the balanced equation for the reaction oxidation: The redox titration of permanganate ions is commonly used to determine the iron content of a. Titration Of Fe2+ With Kmno4 Reaction Equation.
From www.youtube.com
FeC2O4+KMnO4+H2SO4 GIVES Fe2(SO4)3+MnSO4+ K2S04+H2O+CO2. Balancing Titration Of Fe2+ With Kmno4 Reaction Equation It is specifically mentioned in the specification but it could be any redox reaction. In the redox titration between potassium manganate (vii) and fe²⁺ ions the following reactions occur: Write the balanced equation for the reaction oxidation: The ionic equation involved in the process is given below. The redox titration of permanganate ions is commonly used to determine the iron. Titration Of Fe2+ With Kmno4 Reaction Equation.