How To Balance Charges In A Redox Reaction . Balance charge by adding electrons. A) assign oxidation numbers for each atom. To balance a redox reaction, first take an equation and separate into two half reaction equations specifically oxidation and. Balancing simple redox reactions can be a straightforward matter of going back and forth between products and reactants. To balance redox reactions, you must assign oxidation numbers to the reactants and products to determine how many moles of each species are needed to. This requires that one and. B) identify and write out all redox couples in. Updated on september 22, 2019. In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. Learn to balance complex redox reactions by the half reaction method. Learn to balance simple redox reactions by inspection. Write down the unbalanced equation.
from www.youtube.com
Learn to balance simple redox reactions by inspection. Balance charge by adding electrons. Write down the unbalanced equation. This requires that one and. B) identify and write out all redox couples in. To balance redox reactions, you must assign oxidation numbers to the reactants and products to determine how many moles of each species are needed to. Learn to balance complex redox reactions by the half reaction method. Updated on september 22, 2019. To balance a redox reaction, first take an equation and separate into two half reaction equations specifically oxidation and. A) assign oxidation numbers for each atom.
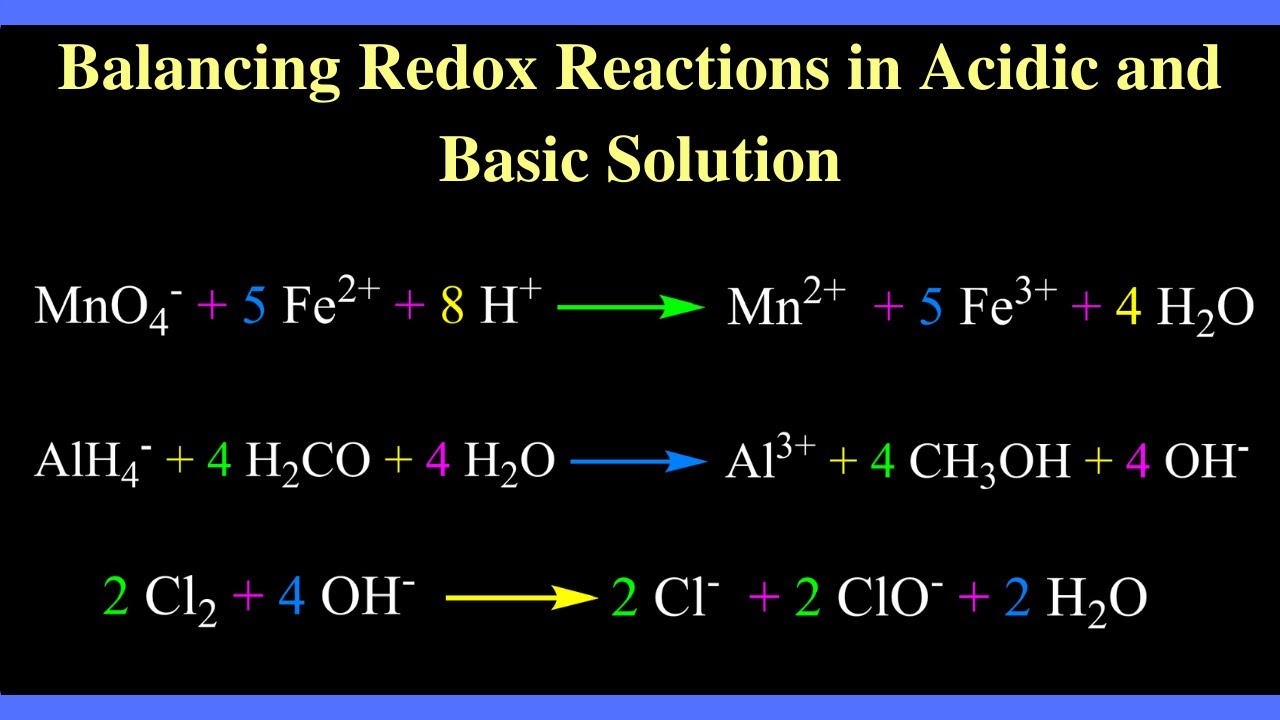
Balancing Redox Reactions in Acidic and Basic Solution YouTube
How To Balance Charges In A Redox Reaction B) identify and write out all redox couples in. A) assign oxidation numbers for each atom. Learn to balance complex redox reactions by the half reaction method. Write down the unbalanced equation. To balance redox reactions, you must assign oxidation numbers to the reactants and products to determine how many moles of each species are needed to. Learn to balance simple redox reactions by inspection. To balance a redox reaction, first take an equation and separate into two half reaction equations specifically oxidation and. Updated on september 22, 2019. In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. B) identify and write out all redox couples in. Balancing simple redox reactions can be a straightforward matter of going back and forth between products and reactants. This requires that one and. Balance charge by adding electrons.
From alevelchemistry.co.uk
Balancing Redox Reactions Facts, Summary & Definition Chemistry How To Balance Charges In A Redox Reaction Updated on september 22, 2019. Write down the unbalanced equation. B) identify and write out all redox couples in. Balancing simple redox reactions can be a straightforward matter of going back and forth between products and reactants. Balance charge by adding electrons. A) assign oxidation numbers for each atom. In a balanced redox reaction, the number of electrons lost by. How To Balance Charges In A Redox Reaction.
From www.youtube.com
How to Balance Redox Equations in Basic Solution YouTube How To Balance Charges In A Redox Reaction To balance redox reactions, you must assign oxidation numbers to the reactants and products to determine how many moles of each species are needed to. Learn to balance complex redox reactions by the half reaction method. B) identify and write out all redox couples in. Balancing simple redox reactions can be a straightforward matter of going back and forth between. How To Balance Charges In A Redox Reaction.
From www.chemistrylearner.com
Redox (OxidationReduction) Reaction Definition & Examples How To Balance Charges In A Redox Reaction Balance charge by adding electrons. Balancing simple redox reactions can be a straightforward matter of going back and forth between products and reactants. A) assign oxidation numbers for each atom. Updated on september 22, 2019. To balance redox reactions, you must assign oxidation numbers to the reactants and products to determine how many moles of each species are needed to.. How To Balance Charges In A Redox Reaction.
From www.youtube.com
How to balance redox reactions in acidic solution YouTube How To Balance Charges In A Redox Reaction In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. A) assign oxidation numbers for each atom. This requires that one and. Balancing simple redox reactions can be a straightforward matter of going back and forth between products and reactants. B) identify and write out all redox couples. How To Balance Charges In A Redox Reaction.
From studylib.net
Balancing Redox Reactions Oxidation Number Method How To Balance Charges In A Redox Reaction A) assign oxidation numbers for each atom. Balance charge by adding electrons. Balancing simple redox reactions can be a straightforward matter of going back and forth between products and reactants. Updated on september 22, 2019. Learn to balance simple redox reactions by inspection. To balance redox reactions, you must assign oxidation numbers to the reactants and products to determine how. How To Balance Charges In A Redox Reaction.
From www.slideserve.com
PPT Balancing Redox Reactions PowerPoint Presentation, free download How To Balance Charges In A Redox Reaction Updated on september 22, 2019. A) assign oxidation numbers for each atom. To balance redox reactions, you must assign oxidation numbers to the reactants and products to determine how many moles of each species are needed to. To balance a redox reaction, first take an equation and separate into two half reaction equations specifically oxidation and. B) identify and write. How To Balance Charges In A Redox Reaction.
From leah4sci.com
Intro to Balancing Redox Reactions How To Balance Charges In A Redox Reaction A) assign oxidation numbers for each atom. To balance a redox reaction, first take an equation and separate into two half reaction equations specifically oxidation and. Balance charge by adding electrons. Learn to balance complex redox reactions by the half reaction method. This requires that one and. Learn to balance simple redox reactions by inspection. In a balanced redox reaction,. How To Balance Charges In A Redox Reaction.
From www.slideserve.com
PPT Aqueoussolution Reactions PowerPoint Presentation ID159152 How To Balance Charges In A Redox Reaction Balance charge by adding electrons. Learn to balance complex redox reactions by the half reaction method. A) assign oxidation numbers for each atom. This requires that one and. Balancing simple redox reactions can be a straightforward matter of going back and forth between products and reactants. B) identify and write out all redox couples in. To balance a redox reaction,. How To Balance Charges In A Redox Reaction.
From worksheetlistee.z21.web.core.windows.net
Balance Redox Reaction Practice How To Balance Charges In A Redox Reaction In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. To balance a redox reaction, first take an equation and separate into two half reaction equations specifically oxidation and. Write down the unbalanced equation. Updated on september 22, 2019. A) assign oxidation numbers for each atom. Learn to. How To Balance Charges In A Redox Reaction.
From www.slideserve.com
PPT Balancing redox reactions PowerPoint Presentation, free download How To Balance Charges In A Redox Reaction Learn to balance simple redox reactions by inspection. This requires that one and. Balance charge by adding electrons. Balancing simple redox reactions can be a straightforward matter of going back and forth between products and reactants. To balance a redox reaction, first take an equation and separate into two half reaction equations specifically oxidation and. Learn to balance complex redox. How To Balance Charges In A Redox Reaction.
From chemistnotes.com
How to Balance Redox Equations Using a Redox Reaction Calculator How To Balance Charges In A Redox Reaction Learn to balance simple redox reactions by inspection. A) assign oxidation numbers for each atom. This requires that one and. Balance charge by adding electrons. Balancing simple redox reactions can be a straightforward matter of going back and forth between products and reactants. B) identify and write out all redox couples in. Updated on september 22, 2019. Write down the. How To Balance Charges In A Redox Reaction.
From www.wikihow.com
How to Balance Redox Reactions (with Pictures) wikiHow How To Balance Charges In A Redox Reaction To balance a redox reaction, first take an equation and separate into two half reaction equations specifically oxidation and. B) identify and write out all redox couples in. Write down the unbalanced equation. Learn to balance complex redox reactions by the half reaction method. Updated on september 22, 2019. This requires that one and. In a balanced redox reaction, the. How To Balance Charges In A Redox Reaction.
From learningchemistryeasily.blogspot.com
Learning Chemistry Easily The Chemical Reaction, Part 5 Balancing a How To Balance Charges In A Redox Reaction In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. Learn to balance complex redox reactions by the half reaction method. To balance redox reactions, you must assign oxidation numbers to the reactants and products to determine how many moles of each species are needed to. This requires. How To Balance Charges In A Redox Reaction.
From chubbyrevision.weebly.com
Chem/Redox Chubby Revision AS Level How To Balance Charges In A Redox Reaction Write down the unbalanced equation. Balance charge by adding electrons. Learn to balance complex redox reactions by the half reaction method. This requires that one and. Balancing simple redox reactions can be a straightforward matter of going back and forth between products and reactants. To balance redox reactions, you must assign oxidation numbers to the reactants and products to determine. How To Balance Charges In A Redox Reaction.
From www.youtube.com
Balancing redox reactions in acid Redox reactions and How To Balance Charges In A Redox Reaction B) identify and write out all redox couples in. A) assign oxidation numbers for each atom. In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. To balance redox reactions, you must assign oxidation numbers to the reactants and products to determine how many moles of each species. How To Balance Charges In A Redox Reaction.
From www.youtube.com
Balancing Redox Reactions in Acidic and Basic Solution YouTube How To Balance Charges In A Redox Reaction Balancing simple redox reactions can be a straightforward matter of going back and forth between products and reactants. This requires that one and. Balance charge by adding electrons. To balance redox reactions, you must assign oxidation numbers to the reactants and products to determine how many moles of each species are needed to. B) identify and write out all redox. How To Balance Charges In A Redox Reaction.
From www.slideserve.com
PPT Balancing Redox Equations PowerPoint Presentation, free download How To Balance Charges In A Redox Reaction A) assign oxidation numbers for each atom. Balancing simple redox reactions can be a straightforward matter of going back and forth between products and reactants. To balance redox reactions, you must assign oxidation numbers to the reactants and products to determine how many moles of each species are needed to. B) identify and write out all redox couples in. Learn. How To Balance Charges In A Redox Reaction.
From www.youtube.com
Balance redox reaction in alkaline medium YouTube How To Balance Charges In A Redox Reaction Balancing simple redox reactions can be a straightforward matter of going back and forth between products and reactants. B) identify and write out all redox couples in. Learn to balance complex redox reactions by the half reaction method. To balance redox reactions, you must assign oxidation numbers to the reactants and products to determine how many moles of each species. How To Balance Charges In A Redox Reaction.
From www.pinterest.com
Tang 02 balancing redox reactions 2 Redox reactions, Chemistry How To Balance Charges In A Redox Reaction To balance a redox reaction, first take an equation and separate into two half reaction equations specifically oxidation and. Balance charge by adding electrons. Learn to balance complex redox reactions by the half reaction method. In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. Updated on september. How To Balance Charges In A Redox Reaction.
From www.youtube.com
Balancing Redox Reactions in Acidic and Basic Conditions YouTube How To Balance Charges In A Redox Reaction A) assign oxidation numbers for each atom. Write down the unbalanced equation. Learn to balance complex redox reactions by the half reaction method. Learn to balance simple redox reactions by inspection. Updated on september 22, 2019. Balancing simple redox reactions can be a straightforward matter of going back and forth between products and reactants. In a balanced redox reaction, the. How To Balance Charges In A Redox Reaction.
From lexiqosnow.blogspot.com
Balancing Net Ionic Redox Equations LexiqoSnow How To Balance Charges In A Redox Reaction Learn to balance simple redox reactions by inspection. A) assign oxidation numbers for each atom. Balance charge by adding electrons. To balance a redox reaction, first take an equation and separate into two half reaction equations specifically oxidation and. This requires that one and. Learn to balance complex redox reactions by the half reaction method. Write down the unbalanced equation.. How To Balance Charges In A Redox Reaction.
From www.youtube.com
Balancing Redox Reactions Half Reaction Method YouTube How To Balance Charges In A Redox Reaction Balance charge by adding electrons. B) identify and write out all redox couples in. Balancing simple redox reactions can be a straightforward matter of going back and forth between products and reactants. A) assign oxidation numbers for each atom. Learn to balance simple redox reactions by inspection. Learn to balance complex redox reactions by the half reaction method. Updated on. How To Balance Charges In A Redox Reaction.
From worksheetlistee.z21.web.core.windows.net
Balance Redox Reaction Example How To Balance Charges In A Redox Reaction In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. This requires that one and. A) assign oxidation numbers for each atom. To balance a redox reaction, first take an equation and separate into two half reaction equations specifically oxidation and. Balance charge by adding electrons. Write down. How To Balance Charges In A Redox Reaction.
From cmc-lubumbashi.com
Balancing redox reactions practice How To Balance Charges In A Redox Reaction To balance redox reactions, you must assign oxidation numbers to the reactants and products to determine how many moles of each species are needed to. Balancing simple redox reactions can be a straightforward matter of going back and forth between products and reactants. B) identify and write out all redox couples in. In a balanced redox reaction, the number of. How To Balance Charges In A Redox Reaction.
From www.wikihow.com
How to Balance Redox Reactions (with Pictures) wikiHow How To Balance Charges In A Redox Reaction Write down the unbalanced equation. This requires that one and. Balance charge by adding electrons. A) assign oxidation numbers for each atom. To balance a redox reaction, first take an equation and separate into two half reaction equations specifically oxidation and. To balance redox reactions, you must assign oxidation numbers to the reactants and products to determine how many moles. How To Balance Charges In A Redox Reaction.
From www.youtube.com
How to balance redox reactions Balancing Redox Reaction Cl2 + OH → How To Balance Charges In A Redox Reaction In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. Balance charge by adding electrons. A) assign oxidation numbers for each atom. To balance redox reactions, you must assign oxidation numbers to the reactants and products to determine how many moles of each species are needed to. B). How To Balance Charges In A Redox Reaction.
From www.slideserve.com
PPT Balancing Oxidation Reduction Equations PowerPoint Presentation How To Balance Charges In A Redox Reaction Write down the unbalanced equation. A) assign oxidation numbers for each atom. Balancing simple redox reactions can be a straightforward matter of going back and forth between products and reactants. Learn to balance complex redox reactions by the half reaction method. To balance redox reactions, you must assign oxidation numbers to the reactants and products to determine how many moles. How To Balance Charges In A Redox Reaction.
From sciencenotes.org
Redox Reactions Identify and Balance Oxidation and Reduction How To Balance Charges In A Redox Reaction B) identify and write out all redox couples in. In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. Learn to balance complex redox reactions by the half reaction method. This requires that one and. Balance charge by adding electrons. Updated on september 22, 2019. Learn to balance. How To Balance Charges In A Redox Reaction.
From www.sliderbase.com
Balancing Chemical Equations. Balancing redox equations Presentation How To Balance Charges In A Redox Reaction Learn to balance simple redox reactions by inspection. In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. Write down the unbalanced equation. Updated on september 22, 2019. B) identify and write out all redox couples in. Balancing simple redox reactions can be a straightforward matter of going. How To Balance Charges In A Redox Reaction.
From cmc-lubumbashi.com
Balancing redox reactions practice How To Balance Charges In A Redox Reaction To balance a redox reaction, first take an equation and separate into two half reaction equations specifically oxidation and. Balancing simple redox reactions can be a straightforward matter of going back and forth between products and reactants. Updated on september 22, 2019. Learn to balance simple redox reactions by inspection. In a balanced redox reaction, the number of electrons lost. How To Balance Charges In A Redox Reaction.
From studylib.net
Balancing Redox Equations Handout How To Balance Charges In A Redox Reaction Learn to balance simple redox reactions by inspection. Balance charge by adding electrons. This requires that one and. Learn to balance complex redox reactions by the half reaction method. To balance redox reactions, you must assign oxidation numbers to the reactants and products to determine how many moles of each species are needed to. B) identify and write out all. How To Balance Charges In A Redox Reaction.
From quizzschoolcartulary.z19.web.core.windows.net
Balance Redox Reaction In Basic Solution How To Balance Charges In A Redox Reaction Updated on september 22, 2019. Balance charge by adding electrons. To balance a redox reaction, first take an equation and separate into two half reaction equations specifically oxidation and. In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. A) assign oxidation numbers for each atom. Learn to. How To Balance Charges In A Redox Reaction.
From www.youtube.com
Balancing Redox Equations using the Oxidation Number Method AP Chem How To Balance Charges In A Redox Reaction Write down the unbalanced equation. A) assign oxidation numbers for each atom. B) identify and write out all redox couples in. This requires that one and. To balance a redox reaction, first take an equation and separate into two half reaction equations specifically oxidation and. Learn to balance complex redox reactions by the half reaction method. Learn to balance simple. How To Balance Charges In A Redox Reaction.
From www.slideserve.com
PPT Balancing Redox Equations following the electrons PowerPoint How To Balance Charges In A Redox Reaction Balancing simple redox reactions can be a straightforward matter of going back and forth between products and reactants. Write down the unbalanced equation. Learn to balance simple redox reactions by inspection. Updated on september 22, 2019. To balance a redox reaction, first take an equation and separate into two half reaction equations specifically oxidation and. Balance charge by adding electrons.. How To Balance Charges In A Redox Reaction.
From www.slideserve.com
PPT 7.3 Balancing Redox Reactions Using Oxidation Numbers How To Balance Charges In A Redox Reaction To balance redox reactions, you must assign oxidation numbers to the reactants and products to determine how many moles of each species are needed to. Learn to balance simple redox reactions by inspection. In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. Write down the unbalanced equation.. How To Balance Charges In A Redox Reaction.