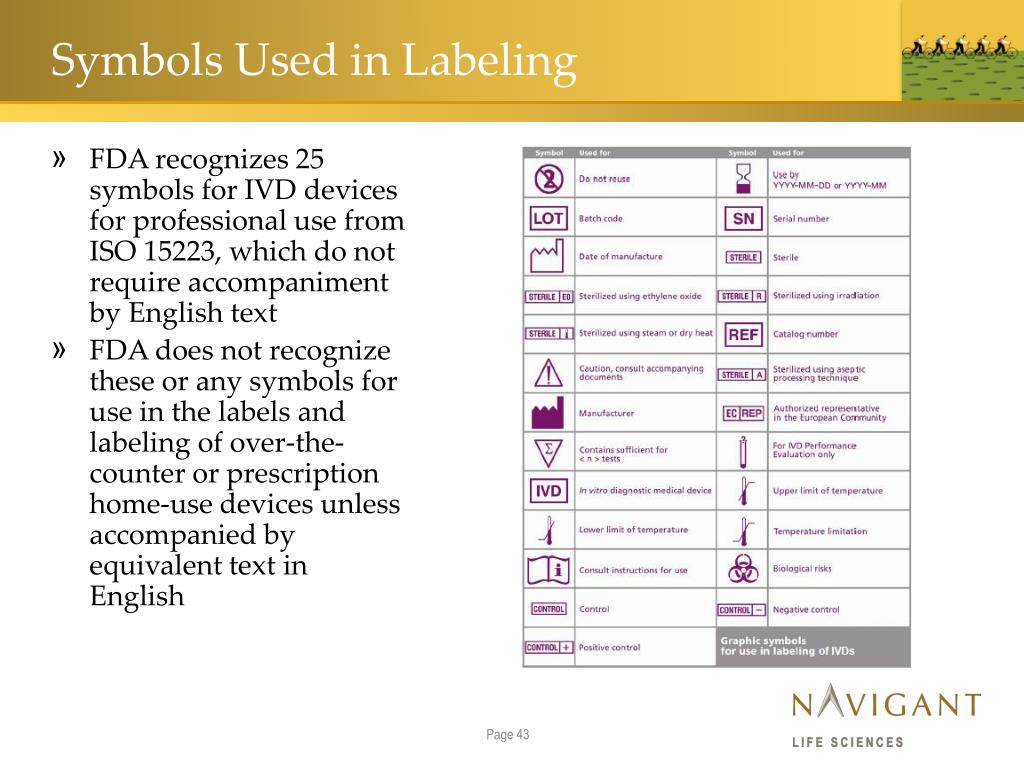
Labelling Standards For Medical Devices . Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. This document specifies the requirements for information supplied by the manufacturer for a medical device or by the. In some jurisdictions, “labelling” is referred to as “information supplied by the manufacturer”. On may 26, 2021, the. This post focuses on medical device labelling requirements and user manual requirements under the eu medical device regulation (mdr) 2017/745. Labelling includes the label, instructions for. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites australia.
from www.slideserve.com
Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites australia. This document specifies the requirements for information supplied by the manufacturer for a medical device or by the. This post focuses on medical device labelling requirements and user manual requirements under the eu medical device regulation (mdr) 2017/745. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Labelling includes the label, instructions for. In some jurisdictions, “labelling” is referred to as “information supplied by the manufacturer”. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. On may 26, 2021, the.
PPT Medical Device Labeling PowerPoint Presentation, free download
Labelling Standards For Medical Devices On may 26, 2021, the. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. This post focuses on medical device labelling requirements and user manual requirements under the eu medical device regulation (mdr) 2017/745. Labelling includes the label, instructions for. This document specifies the requirements for information supplied by the manufacturer for a medical device or by the. On may 26, 2021, the. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites australia. In some jurisdictions, “labelling” is referred to as “information supplied by the manufacturer”.
From peakvascularaccess.com
What is the meaning of symbols on medical devices labels? Peak Mobile Labelling Standards For Medical Devices On may 26, 2021, the. This post focuses on medical device labelling requirements and user manual requirements under the eu medical device regulation (mdr) 2017/745. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Principles of labelling for medical devices. Labelling Standards For Medical Devices.
From vascufirst.com
What is the meaning of symbols on medical devices labels? VascuFirst Labelling Standards For Medical Devices In some jurisdictions, “labelling” is referred to as “information supplied by the manufacturer”. On may 26, 2021, the. This document specifies the requirements for information supplied by the manufacturer for a medical device or by the. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and. Labelling Standards For Medical Devices.
From clin-r.com
Labels for Medical Devices Clin R Labelling Standards For Medical Devices This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. This document specifies the requirements for information supplied by the manufacturer for a medical device or by the. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb). Labelling Standards For Medical Devices.
From www.dreamstime.com
Full Set of International Medical Device Packaging Symbols with Labelling Standards For Medical Devices Labelling includes the label, instructions for. This post focuses on medical device labelling requirements and user manual requirements under the eu medical device regulation (mdr) 2017/745. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. In some jurisdictions, “labelling” is referred to as “information supplied by the manufacturer”.. Labelling Standards For Medical Devices.
From clin-r.com
Labels for Medical Devices Clin R Labelling Standards For Medical Devices This document specifies the requirements for information supplied by the manufacturer for a medical device or by the. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites australia. On may. Labelling Standards For Medical Devices.
From flexlyconsulting.com
MDA New Updates Requirements for Labelling of Medical Devices in Malaysia Labelling Standards For Medical Devices This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites australia. On may 26, 2021, the. This post focuses on medical device labelling. Labelling Standards For Medical Devices.
From clin-r.com
Labels for Medical Devices Clin R Labelling Standards For Medical Devices Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites australia. Labelling includes the label, instructions for. On may 26, 2021, the. This post focuses on medical device labelling requirements and user manual requirements under the eu medical device regulation (mdr) 2017/745. This post will discuss what counts as a medical device. Labelling Standards For Medical Devices.
From www.freseniusmedicalcare.com
Medical device regulation Fresenius Medical Care Labelling Standards For Medical Devices In some jurisdictions, “labelling” is referred to as “information supplied by the manufacturer”. This post focuses on medical device labelling requirements and user manual requirements under the eu medical device regulation (mdr) 2017/745. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites australia. Labelling includes the label, instructions for. Labeling regulations. Labelling Standards For Medical Devices.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Labelling Standards For Medical Devices On may 26, 2021, the. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites australia. Labelling includes the label, instructions for. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Labeling. Labelling Standards For Medical Devices.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Labelling Standards For Medical Devices This document specifies the requirements for information supplied by the manufacturer for a medical device or by the. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb). Labelling Standards For Medical Devices.
From www.slideserve.com
PPT Medical Device Standards PowerPoint Presentation, free download Labelling Standards For Medical Devices This post focuses on medical device labelling requirements and user manual requirements under the eu medical device regulation (mdr) 2017/745. On may 26, 2021, the. Labelling includes the label, instructions for. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations.. Labelling Standards For Medical Devices.
From pharmaknowl.com
SFDA Labelling Requirements PharmaKnowl Labelling Standards For Medical Devices Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites australia. Labelling includes the label, instructions for. In some jurisdictions, “labelling” is referred to as “information supplied by the manufacturer”. This document specifies the requirements for information supplied by the manufacturer for a medical device or by the. On may 26, 2021,. Labelling Standards For Medical Devices.
From e-startupindia.com
US FDA labelling requirements for medical devices EStartupIndia Labelling Standards For Medical Devices This post focuses on medical device labelling requirements and user manual requirements under the eu medical device regulation (mdr) 2017/745. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites australia. This document specifies the requirements for information supplied by the manufacturer for a medical device or by the. In some jurisdictions,. Labelling Standards For Medical Devices.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Labelling Standards For Medical Devices This post focuses on medical device labelling requirements and user manual requirements under the eu medical device regulation (mdr) 2017/745. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites australia. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations.. Labelling Standards For Medical Devices.
From www.qualitymeddev.com
ISO 152232020 Update of for Symbols to be used with Medical Devices Labelling Standards For Medical Devices Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. On may 26, 2021, the. This document specifies the requirements for information supplied by the manufacturer for a medical device or by the. Labelling includes the label, instructions for. In some jurisdictions, “labelling” is referred to as “information supplied. Labelling Standards For Medical Devices.
From www.mavenrs.uk
Medical Devices Labeling Checklist for EU MDR Compliance Maven Labelling Standards For Medical Devices In some jurisdictions, “labelling” is referred to as “information supplied by the manufacturer”. Labelling includes the label, instructions for. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites australia. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. This. Labelling Standards For Medical Devices.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Labelling Standards For Medical Devices This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. This document specifies the requirements for information supplied by the manufacturer for a medical device or by the. This post focuses on medical device labelling requirements and user manual requirements under. Labelling Standards For Medical Devices.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Labelling Standards For Medical Devices On may 26, 2021, the. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. This document specifies the requirements for information supplied by the manufacturer for a medical device or by the. Labelling includes the label, instructions for. This post. Labelling Standards For Medical Devices.
From www.scilife.io
Labeling Requirements for Medical Devices Scilife Labelling Standards For Medical Devices Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites australia. In some jurisdictions, “labelling” is referred to as “information supplied by the manufacturer”. On may 26, 2021, the. This document specifies the requirements for information supplied by the manufacturer for a medical device or by the. This post will discuss what. Labelling Standards For Medical Devices.
From www.en-standard.eu
BS EN 159862011 Symbol for use in the labelling of medical devices Labelling Standards For Medical Devices Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites australia. Labelling includes the label, instructions for. On may 26, 2021, the. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Labeling. Labelling Standards For Medical Devices.
From knconsultingandservices.com
What is Labelling? Medical Device Consulting Company Labelling Standards For Medical Devices On may 26, 2021, the. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites australia. This post focuses on medical device labelling requirements and user manual requirements under the eu medical device regulation (mdr) 2017/745. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of. Labelling Standards For Medical Devices.
From vascufirst.com
What is the meaning of symbols on medical devices labels? VascuFirst Labelling Standards For Medical Devices Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Labelling includes the label, instructions for. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. This document specifies the. Labelling Standards For Medical Devices.
From www.regdesk.co
HSA Guidance on Labeling for Medical Devices Introduction RegDesk Labelling Standards For Medical Devices Labelling includes the label, instructions for. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites australia. On may 26, 2021, the. This document specifies the requirements for information supplied by the manufacturer for a medical device or by the. In some jurisdictions, “labelling” is referred to as “information supplied by the. Labelling Standards For Medical Devices.
From dandelionsandthings.blogspot.com
30 Medical Device Label Symbols Label Design Ideas 2020 Labelling Standards For Medical Devices Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites australia. This document specifies the requirements for information supplied by the manufacturer for a medical device or by the. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. On may. Labelling Standards For Medical Devices.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Labelling Standards For Medical Devices This document specifies the requirements for information supplied by the manufacturer for a medical device or by the. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. In some jurisdictions, “labelling” is referred to as “information supplied by the manufacturer”.. Labelling Standards For Medical Devices.
From mavink.com
Medical Device Labeling Symbols Labelling Standards For Medical Devices Labelling includes the label, instructions for. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites australia. This document specifies the requirements for. Labelling Standards For Medical Devices.
From www.afpharmaservice.com
Medical Device Labelling Requirements Labelling Standards For Medical Devices Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites australia. This post focuses on medical device labelling requirements and user manual requirements under the eu medical device regulation (mdr) 2017/745. This document specifies the requirements for information supplied by the manufacturer for a medical device or by the. This post will. Labelling Standards For Medical Devices.
From www.flexo-graphics.com
Medical Device Labeling Medical Equipment Labels Labelling Standards For Medical Devices On may 26, 2021, the. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites australia. In some jurisdictions, “labelling” is referred to as “information supplied by the manufacturer”. This post focuses on medical device labelling requirements and user manual requirements under the eu medical device regulation (mdr) 2017/745. Labeling regulations pertaining. Labelling Standards For Medical Devices.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Labelling Standards For Medical Devices In some jurisdictions, “labelling” is referred to as “information supplied by the manufacturer”. Labelling includes the label, instructions for. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. This post will discuss what counts as a medical device label, where they are required, and look at the key. Labelling Standards For Medical Devices.
From www.schlafenderhase.com
Medical Device Labeling Requirements Schlafender Hase Labelling Standards For Medical Devices On may 26, 2021, the. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. This document specifies the requirements for information supplied by the manufacturer for a medical device or by the. In some jurisdictions, “labelling” is referred to as “information supplied by the manufacturer”. Principles of labelling. Labelling Standards For Medical Devices.
From cliniexperts.com
MoHFW Notification For Medical Devices Registration & Labelling, India Labelling Standards For Medical Devices On may 26, 2021, the. In some jurisdictions, “labelling” is referred to as “information supplied by the manufacturer”. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites australia. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us. Labelling Standards For Medical Devices.
From www.vrogue.co
Eu Mdr Medical Device Labeling Requirements A Complet vrogue.co Labelling Standards For Medical Devices Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. On may 26, 2021, the. Principles of labelling for medical. Labelling Standards For Medical Devices.
From operonstrategist.com
eIFU in medical devices & labelling for medical devices as per EUMDR Labelling Standards For Medical Devices Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites australia. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. This document specifies the requirements for information supplied by the manufacturer for. Labelling Standards For Medical Devices.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Labelling Standards For Medical Devices This document specifies the requirements for information supplied by the manufacturer for a medical device or by the. In some jurisdictions, “labelling” is referred to as “information supplied by the manufacturer”. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. This post will discuss what counts as a. Labelling Standards For Medical Devices.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Labelling Standards For Medical Devices This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. In some jurisdictions, “labelling” is referred to as “information supplied by the manufacturer”. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Labelling Standards For Medical Devices.