Copper In A Brass Alloy Lab . You will dissolve the brass in nitric. Since alloys are mixtures, their composition can vary widely. Brass can be dissolved by reacting it with. The purpose of this lab is to analyze the amount of copper in brass using visible spectroscopy. It is an alloy, contains copper and zinc as major constituents. In this experiment you will be finding out how much copper there is in brass (an alloy of copper and zinc). One example of an alloy is brass, which is composed primarily of copper (cu) and zinc (zn). After complete dissolution ∼ of. Depending upon the proportions of copper and zinc the. It should take approximately 25 minutes. In this experiment, students determine the copper content in brass (an alloy of copper and zinc) by dissolving brass turnings in nitric acid and comparing the colour of the solution with that of solutions of various concentrations of copper. The objective of this investigation is to determine how much copper is in a sample of brass. By treating a brass sample with nitric acid, the. Some brasses also have small.
from www.chegg.com
It should take approximately 25 minutes. Some brasses also have small. Depending upon the proportions of copper and zinc the. Brass can be dissolved by reacting it with. In this experiment you will be finding out how much copper there is in brass (an alloy of copper and zinc). The objective of this investigation is to determine how much copper is in a sample of brass. After complete dissolution ∼ of. By treating a brass sample with nitric acid, the. It is an alloy, contains copper and zinc as major constituents. You will dissolve the brass in nitric.
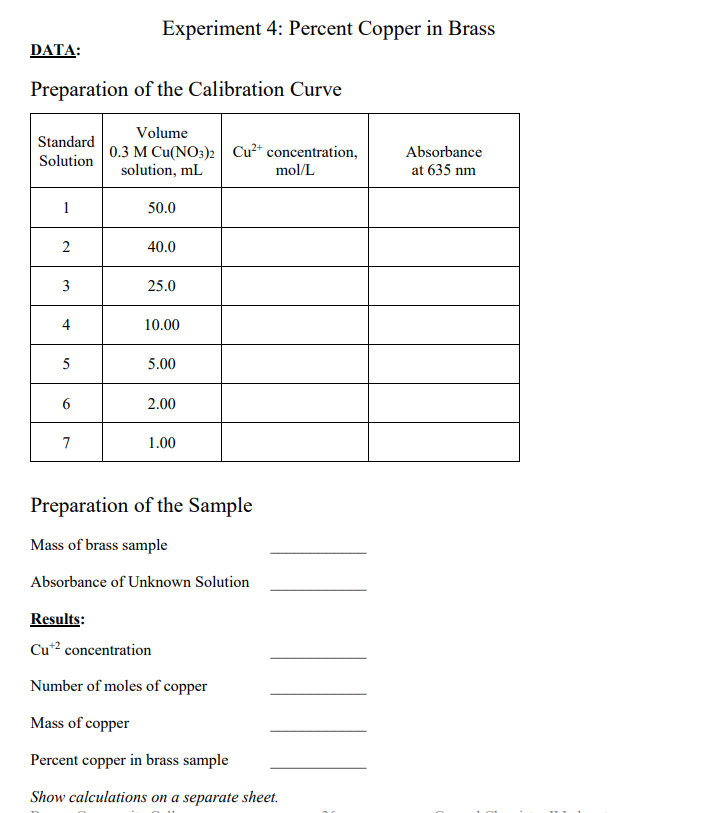
This is a chemistry lab for Percent Copper in Brass.
Copper In A Brass Alloy Lab The purpose of this lab is to analyze the amount of copper in brass using visible spectroscopy. The purpose of this lab is to analyze the amount of copper in brass using visible spectroscopy. In this experiment you will be finding out how much copper there is in brass (an alloy of copper and zinc). Since alloys are mixtures, their composition can vary widely. Depending upon the proportions of copper and zinc the. You will dissolve the brass in nitric. One example of an alloy is brass, which is composed primarily of copper (cu) and zinc (zn). After complete dissolution ∼ of. It should take approximately 25 minutes. In this experiment, students determine the copper content in brass (an alloy of copper and zinc) by dissolving brass turnings in nitric acid and comparing the colour of the solution with that of solutions of various concentrations of copper. Brass can be dissolved by reacting it with. It is an alloy, contains copper and zinc as major constituents. The objective of this investigation is to determine how much copper is in a sample of brass. By treating a brass sample with nitric acid, the. Some brasses also have small.
From sciencenotes.org
What Is Brass Made Of? Difference Between Brass and Bronze Copper In A Brass Alloy Lab In this experiment, students determine the copper content in brass (an alloy of copper and zinc) by dissolving brass turnings in nitric acid and comparing the colour of the solution with that of solutions of various concentrations of copper. In this experiment you will be finding out how much copper there is in brass (an alloy of copper and zinc).. Copper In A Brass Alloy Lab.
From huazhusteel.en.made-in-china.com
Copper Brass Grades Alloys ASTM C10100 C11000 0.88mm Sheet / Plate/for Copper In A Brass Alloy Lab One example of an alloy is brass, which is composed primarily of copper (cu) and zinc (zn). The objective of this investigation is to determine how much copper is in a sample of brass. It is an alloy, contains copper and zinc as major constituents. Brass can be dissolved by reacting it with. By treating a brass sample with nitric. Copper In A Brass Alloy Lab.
From www.youtube.com
Percent Copper in Brass Lab YouTube Copper In A Brass Alloy Lab Brass can be dissolved by reacting it with. You will dissolve the brass in nitric. By treating a brass sample with nitric acid, the. After complete dissolution ∼ of. The objective of this investigation is to determine how much copper is in a sample of brass. In this experiment you will be finding out how much copper there is in. Copper In A Brass Alloy Lab.
From www.copper.org
Resources Standards & Properties Copper & Copper Alloy Copper In A Brass Alloy Lab The purpose of this lab is to analyze the amount of copper in brass using visible spectroscopy. You will dissolve the brass in nitric. Since alloys are mixtures, their composition can vary widely. Some brasses also have small. Depending upon the proportions of copper and zinc the. Brass can be dissolved by reacting it with. The objective of this investigation. Copper In A Brass Alloy Lab.
From blog.thepipingmart.com
CopperZinc Alloys A Complete Guide Copper In A Brass Alloy Lab After complete dissolution ∼ of. The objective of this investigation is to determine how much copper is in a sample of brass. It is an alloy, contains copper and zinc as major constituents. Some brasses also have small. One example of an alloy is brass, which is composed primarily of copper (cu) and zinc (zn). Depending upon the proportions of. Copper In A Brass Alloy Lab.
From www.chegg.com
This is a chemistry lab for Percent Copper in Brass. Copper In A Brass Alloy Lab Some brasses also have small. Brass can be dissolved by reacting it with. By treating a brass sample with nitric acid, the. After complete dissolution ∼ of. One example of an alloy is brass, which is composed primarily of copper (cu) and zinc (zn). It is an alloy, contains copper and zinc as major constituents. You will dissolve the brass. Copper In A Brass Alloy Lab.
From www.chegg.com
Solved 10 14. 3. 7. 16, 1. Brass is an alloy of copper and Copper In A Brass Alloy Lab After complete dissolution ∼ of. Some brasses also have small. It is an alloy, contains copper and zinc as major constituents. In this experiment you will be finding out how much copper there is in brass (an alloy of copper and zinc). One example of an alloy is brass, which is composed primarily of copper (cu) and zinc (zn). Brass. Copper In A Brass Alloy Lab.
From exoqwgizi.blob.core.windows.net
Brass Alloy Color at Judy Meyer blog Copper In A Brass Alloy Lab The objective of this investigation is to determine how much copper is in a sample of brass. Brass can be dissolved by reacting it with. One example of an alloy is brass, which is composed primarily of copper (cu) and zinc (zn). After complete dissolution ∼ of. By treating a brass sample with nitric acid, the. In this experiment, students. Copper In A Brass Alloy Lab.
From blog.thepipingmart.com
Advantages and Disadvantages of Brass Over Copper Copper In A Brass Alloy Lab In this experiment, students determine the copper content in brass (an alloy of copper and zinc) by dissolving brass turnings in nitric acid and comparing the colour of the solution with that of solutions of various concentrations of copper. One example of an alloy is brass, which is composed primarily of copper (cu) and zinc (zn). The purpose of this. Copper In A Brass Alloy Lab.
From www.copper.org
Standards & Properties Mechanical Properties of Copper and Copper Copper In A Brass Alloy Lab It should take approximately 25 minutes. Some brasses also have small. One example of an alloy is brass, which is composed primarily of copper (cu) and zinc (zn). Since alloys are mixtures, their composition can vary widely. You will dissolve the brass in nitric. In this experiment you will be finding out how much copper there is in brass (an. Copper In A Brass Alloy Lab.
From www.chegg.com
This is a chemistry lab for Percent Copper in Brass. Copper In A Brass Alloy Lab By treating a brass sample with nitric acid, the. It should take approximately 25 minutes. Brass can be dissolved by reacting it with. You will dissolve the brass in nitric. Depending upon the proportions of copper and zinc the. After complete dissolution ∼ of. It is an alloy, contains copper and zinc as major constituents. In this experiment, students determine. Copper In A Brass Alloy Lab.
From dwdbrass.com
Copper Alloy Brass Tubes, Copper Pipes Copper In A Brass Alloy Lab In this experiment you will be finding out how much copper there is in brass (an alloy of copper and zinc). The objective of this investigation is to determine how much copper is in a sample of brass. In this experiment, students determine the copper content in brass (an alloy of copper and zinc) by dissolving brass turnings in nitric. Copper In A Brass Alloy Lab.
From www.flinnsci.com
FlinnPREP™ Inquiry Labs for AP® Chemistry Percent Copper in Brass Copper In A Brass Alloy Lab In this experiment, students determine the copper content in brass (an alloy of copper and zinc) by dissolving brass turnings in nitric acid and comparing the colour of the solution with that of solutions of various concentrations of copper. You will dissolve the brass in nitric. Since alloys are mixtures, their composition can vary widely. It is an alloy, contains. Copper In A Brass Alloy Lab.
From www.chemedx.org
An Easy Copper Electroplating Demo for Your Redox Unit Chemical Copper In A Brass Alloy Lab The purpose of this lab is to analyze the amount of copper in brass using visible spectroscopy. Depending upon the proportions of copper and zinc the. By treating a brass sample with nitric acid, the. You will dissolve the brass in nitric. One example of an alloy is brass, which is composed primarily of copper (cu) and zinc (zn). In. Copper In A Brass Alloy Lab.
From blog.thepipingmart.com
7 Types Of Copper Alloys And Their Uses Copper In A Brass Alloy Lab One example of an alloy is brass, which is composed primarily of copper (cu) and zinc (zn). After complete dissolution ∼ of. In this experiment you will be finding out how much copper there is in brass (an alloy of copper and zinc). Brass can be dissolved by reacting it with. Depending upon the proportions of copper and zinc the.. Copper In A Brass Alloy Lab.
From blog.thepipingmart.com
Copper Alloy Properties Physical, Chemical, Mechanical Copper In A Brass Alloy Lab Brass can be dissolved by reacting it with. It should take approximately 25 minutes. Since alloys are mixtures, their composition can vary widely. One example of an alloy is brass, which is composed primarily of copper (cu) and zinc (zn). Some brasses also have small. In this experiment you will be finding out how much copper there is in brass. Copper In A Brass Alloy Lab.
From lulelaboratory.blogspot.com
Lu Le Laboratory The Determination of Copper in Brass Lu Le Laboratory Copper In A Brass Alloy Lab In this experiment, students determine the copper content in brass (an alloy of copper and zinc) by dissolving brass turnings in nitric acid and comparing the colour of the solution with that of solutions of various concentrations of copper. The purpose of this lab is to analyze the amount of copper in brass using visible spectroscopy. The objective of this. Copper In A Brass Alloy Lab.
From www.meadmetals.com
A Guide to Copper Alloys Brass vs. Phosphor Bronze Copper In A Brass Alloy Lab Some brasses also have small. The objective of this investigation is to determine how much copper is in a sample of brass. One example of an alloy is brass, which is composed primarily of copper (cu) and zinc (zn). Depending upon the proportions of copper and zinc the. Brass can be dissolved by reacting it with. The purpose of this. Copper In A Brass Alloy Lab.
From www.copper.org
Resources Standards & Properties Copper & Copper Alloy Copper In A Brass Alloy Lab It is an alloy, contains copper and zinc as major constituents. The purpose of this lab is to analyze the amount of copper in brass using visible spectroscopy. In this experiment, students determine the copper content in brass (an alloy of copper and zinc) by dissolving brass turnings in nitric acid and comparing the colour of the solution with that. Copper In A Brass Alloy Lab.
From sciencing.com
How to Find the Percentage of Copper in a Brass Alloy Assignment Copper In A Brass Alloy Lab After complete dissolution ∼ of. It is an alloy, contains copper and zinc as major constituents. Depending upon the proportions of copper and zinc the. It should take approximately 25 minutes. Since alloys are mixtures, their composition can vary widely. In this experiment, students determine the copper content in brass (an alloy of copper and zinc) by dissolving brass turnings. Copper In A Brass Alloy Lab.
From dwdbrass.com
Copper Alloys before Using a Brass Angle or Bar for Your Project Copper In A Brass Alloy Lab One example of an alloy is brass, which is composed primarily of copper (cu) and zinc (zn). It should take approximately 25 minutes. You will dissolve the brass in nitric. The objective of this investigation is to determine how much copper is in a sample of brass. After complete dissolution ∼ of. The purpose of this lab is to analyze. Copper In A Brass Alloy Lab.
From edu.rsc.org
The determination of copper in brass Experiment RSC Education Copper In A Brass Alloy Lab Some brasses also have small. In this experiment you will be finding out how much copper there is in brass (an alloy of copper and zinc). Depending upon the proportions of copper and zinc the. Since alloys are mixtures, their composition can vary widely. It should take approximately 25 minutes. You will dissolve the brass in nitric. The purpose of. Copper In A Brass Alloy Lab.
From www.metaltek.com
Copper vs. Brass vs. Bronze The Difference Between Alloys MetalTek Copper In A Brass Alloy Lab By treating a brass sample with nitric acid, the. It is an alloy, contains copper and zinc as major constituents. After complete dissolution ∼ of. Brass can be dissolved by reacting it with. The objective of this investigation is to determine how much copper is in a sample of brass. It should take approximately 25 minutes. Some brasses also have. Copper In A Brass Alloy Lab.
From dwdbrass.com
Copper and Copper Alloys Tubes Brass Tubes, Copper Pipes Copper In A Brass Alloy Lab The objective of this investigation is to determine how much copper is in a sample of brass. It should take approximately 25 minutes. In this experiment, students determine the copper content in brass (an alloy of copper and zinc) by dissolving brass turnings in nitric acid and comparing the colour of the solution with that of solutions of various concentrations. Copper In A Brass Alloy Lab.
From www.slideserve.com
PPT COPPER ALLOYS PowerPoint Presentation, free download ID6094985 Copper In A Brass Alloy Lab It should take approximately 25 minutes. In this experiment you will be finding out how much copper there is in brass (an alloy of copper and zinc). Since alloys are mixtures, their composition can vary widely. Some brasses also have small. You will dissolve the brass in nitric. Brass can be dissolved by reacting it with. After complete dissolution ∼. Copper In A Brass Alloy Lab.
From localrevive.com
Metallography of copper and copper alloys insight (2023) Copper In A Brass Alloy Lab By treating a brass sample with nitric acid, the. After complete dissolution ∼ of. The objective of this investigation is to determine how much copper is in a sample of brass. You will dissolve the brass in nitric. Brass can be dissolved by reacting it with. Since alloys are mixtures, their composition can vary widely. In this experiment you will. Copper In A Brass Alloy Lab.
From www.chegg.com
Experiment 4 Percent Copper in Brass OBJECTIVE To Copper In A Brass Alloy Lab The purpose of this lab is to analyze the amount of copper in brass using visible spectroscopy. One example of an alloy is brass, which is composed primarily of copper (cu) and zinc (zn). Brass can be dissolved by reacting it with. By treating a brass sample with nitric acid, the. Some brasses also have small. After complete dissolution ∼. Copper In A Brass Alloy Lab.
From www.youtube.com
Mixing Metals Casting Challenge Melting Copper, Brass, And Aluminum Copper In A Brass Alloy Lab After complete dissolution ∼ of. In this experiment you will be finding out how much copper there is in brass (an alloy of copper and zinc). Depending upon the proportions of copper and zinc the. In this experiment, students determine the copper content in brass (an alloy of copper and zinc) by dissolving brass turnings in nitric acid and comparing. Copper In A Brass Alloy Lab.
From blog.thepipingmart.com
CopperZinc Alloys An Overview Copper In A Brass Alloy Lab You will dissolve the brass in nitric. It is an alloy, contains copper and zinc as major constituents. Since alloys are mixtures, their composition can vary widely. Brass can be dissolved by reacting it with. In this experiment you will be finding out how much copper there is in brass (an alloy of copper and zinc). The purpose of this. Copper In A Brass Alloy Lab.
From informacionpublica.svet.gob.gt
Brass CopperZinc Alloys Properties And Uses Copper In A Brass Alloy Lab It should take approximately 25 minutes. By treating a brass sample with nitric acid, the. The objective of this investigation is to determine how much copper is in a sample of brass. It is an alloy, contains copper and zinc as major constituents. Some brasses also have small. In this experiment, students determine the copper content in brass (an alloy. Copper In A Brass Alloy Lab.
From shenghuigangtie.en.made-in-china.com
China Copper Factory T2 Pure Red Copper Strip H62 C27200 Brass Copper Copper In A Brass Alloy Lab You will dissolve the brass in nitric. Some brasses also have small. The purpose of this lab is to analyze the amount of copper in brass using visible spectroscopy. The objective of this investigation is to determine how much copper is in a sample of brass. In this experiment, students determine the copper content in brass (an alloy of copper. Copper In A Brass Alloy Lab.
From xometry.eu
Copper and brass grades crossreference of designation standards Copper In A Brass Alloy Lab Some brasses also have small. By treating a brass sample with nitric acid, the. In this experiment, students determine the copper content in brass (an alloy of copper and zinc) by dissolving brass turnings in nitric acid and comparing the colour of the solution with that of solutions of various concentrations of copper. After complete dissolution ∼ of. The objective. Copper In A Brass Alloy Lab.
From www.chegg.com
Solved South Pasadena AP Chemistry 1 Describing Matter Name Copper In A Brass Alloy Lab It is an alloy, contains copper and zinc as major constituents. After complete dissolution ∼ of. By treating a brass sample with nitric acid, the. Brass can be dissolved by reacting it with. The purpose of this lab is to analyze the amount of copper in brass using visible spectroscopy. You will dissolve the brass in nitric. Depending upon the. Copper In A Brass Alloy Lab.
From dwdbrass.com
RWMA COPPER ALLOYS Brass Tubes, Copper Pipes Copper In A Brass Alloy Lab Some brasses also have small. You will dissolve the brass in nitric. It is an alloy, contains copper and zinc as major constituents. One example of an alloy is brass, which is composed primarily of copper (cu) and zinc (zn). Depending upon the proportions of copper and zinc the. By treating a brass sample with nitric acid, the. The purpose. Copper In A Brass Alloy Lab.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID1206242 Copper In A Brass Alloy Lab It should take approximately 25 minutes. In this experiment you will be finding out how much copper there is in brass (an alloy of copper and zinc). The purpose of this lab is to analyze the amount of copper in brass using visible spectroscopy. You will dissolve the brass in nitric. After complete dissolution ∼ of. One example of an. Copper In A Brass Alloy Lab.