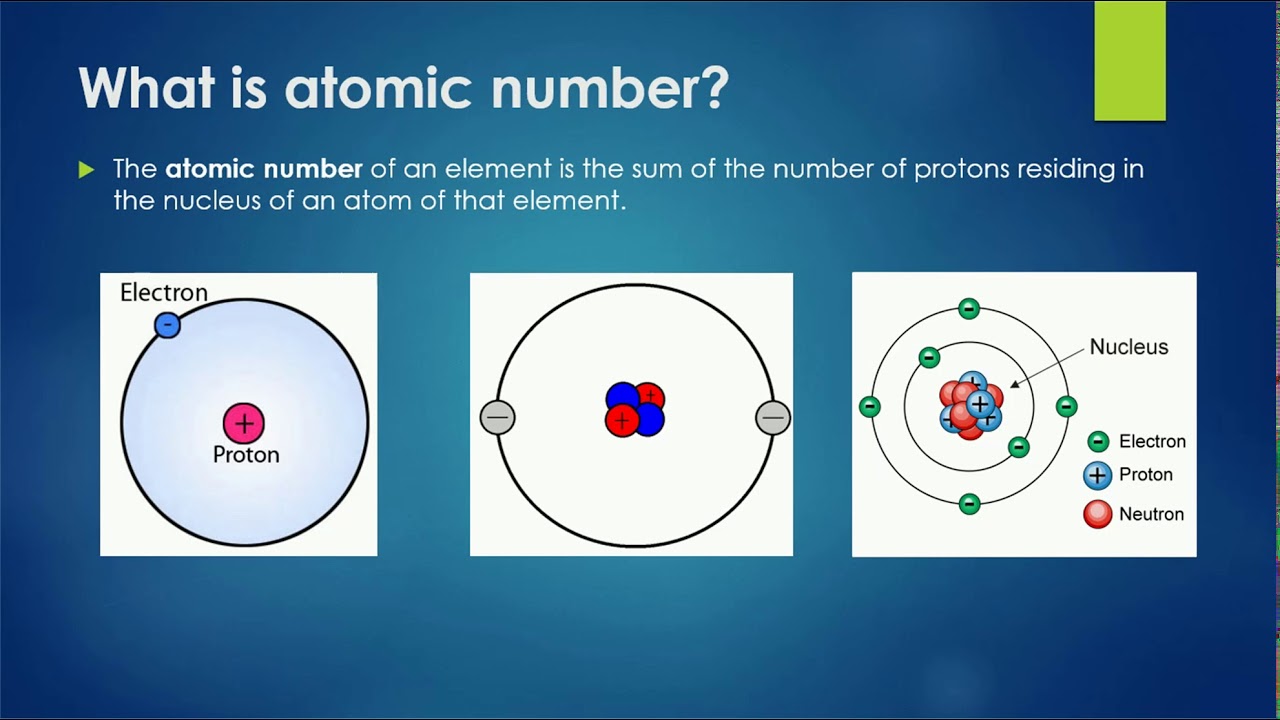
What Is The Atomic Number For Z . Elements are different because of their atomic number. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. For example, the atomic number (z) for sodium (na) is 11. This means that the number of protons is the characteristic which makes each element unique compared to all other elements. An atom can be classified as a particular. The symbol for the atomic number is designated with the letter z. The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element. The atomic number is the number of protons found in the nucleus of an atom, which uniquely identifies its element. Prior to 1915, the symbol z denoted the position. The symbol for atomic number, z, stands for zahl, which means number in german. That means that all sodium atoms have 11 protons. The atomic number is also called the proton number.
from www.youtube.com
That means that all sodium atoms have 11 protons. Elements are different because of their atomic number. This means that the number of protons is the characteristic which makes each element unique compared to all other elements. The symbol for atomic number, z, stands for zahl, which means number in german. The atomic number is the number of protons found in the nucleus of an atom, which uniquely identifies its element. The symbol for the atomic number is designated with the letter z. The atomic number is also called the proton number. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. An atom can be classified as a particular.
What is Atomic Number? Examples Chemistry YouTube
What Is The Atomic Number For Z This means that the number of protons is the characteristic which makes each element unique compared to all other elements. The atomic number is the number of protons found in the nucleus of an atom, which uniquely identifies its element. This means that the number of protons is the characteristic which makes each element unique compared to all other elements. That means that all sodium atoms have 11 protons. For example, the atomic number (z) for sodium (na) is 11. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element. The atomic number is also called the proton number. The symbol for the atomic number is designated with the letter z. The symbol for atomic number, z, stands for zahl, which means number in german. Prior to 1915, the symbol z denoted the position. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. An atom can be classified as a particular. The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. Elements are different because of their atomic number.
From www.slideserve.com
PPT Atomic Notation PowerPoint Presentation, free download ID2978501 What Is The Atomic Number For Z The symbol for atomic number, z, stands for zahl, which means number in german. The atomic number is also called the proton number. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element. For example, the atomic number (z) for sodium (na) is 11. This. What Is The Atomic Number For Z.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation, free download ID4825731 What Is The Atomic Number For Z The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element. An atom can be classified as a particular. The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. Prior to 1915, the symbol z. What Is The Atomic Number For Z.
From www.slideserve.com
PPT Atomic Number, Mass Number, Atomic Mass and Isotopes PowerPoint What Is The Atomic Number For Z The symbol for atomic number, z, stands for zahl, which means number in german. For example, the atomic number (z) for sodium (na) is 11. An atom can be classified as a particular. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element. The atomic. What Is The Atomic Number For Z.
From www.britannica.com
Elements of the Periodic Table Quiz What Is The Atomic Number For Z Elements are different because of their atomic number. That means that all sodium atoms have 11 protons. The atomic number is the number of protons found in the nucleus of an atom, which uniquely identifies its element. The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. Prior to. What Is The Atomic Number For Z.
From slideplayer.com
The Atom, the Nucleus and Radioactivity ppt download What Is The Atomic Number For Z 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. The atomic number is the number of protons found in the nucleus of an atom, which uniquely identifies its element. Elements are different because of their atomic number. The symbol for atomic number, z, stands for zahl, which means number in german.. What Is The Atomic Number For Z.
From www.youtube.com
How to Find the Atomic Number on the Periodic Table YouTube What Is The Atomic Number For Z The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. The symbol for atomic number, z, stands for zahl, which means number in german. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element.. What Is The Atomic Number For Z.
From www.numerade.com
SOLVEDFind the atomic number (Z) for each element. (a) Si (b) W (c) Ni What Is The Atomic Number For Z The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. That means that all sodium atoms have 11 protons. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element. For example, the atomic number. What Is The Atomic Number For Z.
From sciencenotes.org
Nuclear Symbol Notation What Is The Atomic Number For Z The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. This means that the number of protons is the characteristic which makes each element unique compared to all other elements. Prior to 1915, the symbol z denoted the position. The atomic number (represented by the letter z) of an. What Is The Atomic Number For Z.
From journeyz.co
What Are the Three Parts of an Atom? What Is The Atomic Number For Z This means that the number of protons is the characteristic which makes each element unique compared to all other elements. The symbol for atomic number, z, stands for zahl, which means number in german. The symbol for the atomic number is designated with the letter z. 119 rows here is a list of elements of the periodic table, their atomic. What Is The Atomic Number For Z.
From periodictable.me
Atomic Number Examples Archives Dynamic Periodic Table of Elements What Is The Atomic Number For Z The atomic number is the number of protons found in the nucleus of an atom, which uniquely identifies its element. The atomic number is also called the proton number. Prior to 1915, the symbol z denoted the position. The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. That. What Is The Atomic Number For Z.
From revisionscience.com
Atomic Number and Mass Number ALevel Chemistry What Is The Atomic Number For Z The atomic number is also called the proton number. Prior to 1915, the symbol z denoted the position. For example, the atomic number (z) for sodium (na) is 11. That means that all sodium atoms have 11 protons. The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. Elements. What Is The Atomic Number For Z.
From www.vectorstock.com
Periodic table of the elements with atomic number Vector Image What Is The Atomic Number For Z This means that the number of protons is the characteristic which makes each element unique compared to all other elements. Prior to 1915, the symbol z denoted the position. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element. The atomic number (z) of an. What Is The Atomic Number For Z.
From www.slideserve.com
PPT Atomic Notation PowerPoint Presentation, free download ID2978501 What Is The Atomic Number For Z 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. That means that all sodium atoms have 11 protons. Prior to 1915, the symbol z denoted the position. This means that the number of protons is the characteristic which makes each element unique compared to all other elements. The atomic number is. What Is The Atomic Number For Z.
From www.thoughtco.com
Element List Atomic Number, Element Name and Symbol What Is The Atomic Number For Z The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. The symbol for atomic number, z, stands for zahl, which means number in german. The atomic number is the number of protons found in the nucleus of an atom, which uniquely identifies its element. For example, the atomic number. What Is The Atomic Number For Z.
From www.expii.com
Atomic Number — Definition & Overview Expii What Is The Atomic Number For Z Prior to 1915, the symbol z denoted the position. This means that the number of protons is the characteristic which makes each element unique compared to all other elements. The atomic number is also called the proton number. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. The symbol for the. What Is The Atomic Number For Z.
From www.slideserve.com
PPT AHS Chemistry Unit 2 Atomic Structure PowerPoint Presentation What Is The Atomic Number For Z This means that the number of protons is the characteristic which makes each element unique compared to all other elements. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. An atom can be classified as a particular. Elements are different because of their atomic number. The symbol for atomic number, z,. What Is The Atomic Number For Z.
From www.slideserve.com
PPT Atoms and ElementsThe Nature of Matter PowerPoint Presentation What Is The Atomic Number For Z Prior to 1915, the symbol z denoted the position. This means that the number of protons is the characteristic which makes each element unique compared to all other elements. Elements are different because of their atomic number. The atomic number is the number of protons found in the nucleus of an atom, which uniquely identifies its element. For example, the. What Is The Atomic Number For Z.
From www.youtube.com
What Is Atomic Number/Why Atomic Number Is Represented By (Z) YouTube What Is The Atomic Number For Z An atom can be classified as a particular. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. The atomic number (represented by the letter z) of an element is the. What Is The Atomic Number For Z.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation, free download ID6550525 What Is The Atomic Number For Z The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. The atomic number is the number of protons found in the nucleus of an atom, which uniquely identifies its element. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus. What Is The Atomic Number For Z.
From www.slideserve.com
PPT Atomic Theory PowerPoint Presentation, free download ID4938846 What Is The Atomic Number For Z The atomic number is also called the proton number. Elements are different because of their atomic number. The atomic number is the number of protons found in the nucleus of an atom, which uniquely identifies its element. That means that all sodium atoms have 11 protons. The atomic number (z) of an element is the number of protons in the. What Is The Atomic Number For Z.
From sciencenotes.org
List of Elements By Atomic Number What Is The Atomic Number For Z Prior to 1915, the symbol z denoted the position. The atomic number is also called the proton number. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element. That means that all sodium atoms have 11 protons. The symbol for atomic number, z, stands for. What Is The Atomic Number For Z.
From utedzz.blogspot.com
Periodic Table With Names Of Elements And Symbols Periodic Table Timeline What Is The Atomic Number For Z Elements are different because of their atomic number. This means that the number of protons is the characteristic which makes each element unique compared to all other elements. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element. 119 rows here is a list of. What Is The Atomic Number For Z.
From www.worksheetsplanet.com
What is the Atomic Number? Worksheets What Is The Atomic Number For Z The symbol for the atomic number is designated with the letter z. Elements are different because of their atomic number. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element. This means that the number of protons is the characteristic which makes each element unique. What Is The Atomic Number For Z.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation, free download ID5850432 What Is The Atomic Number For Z The atomic number is also called the proton number. Elements are different because of their atomic number. An atom can be classified as a particular. This means that the number of protons is the characteristic which makes each element unique compared to all other elements. The atomic number (represented by the letter z) of an element is the number of. What Is The Atomic Number For Z.
From www.slideserve.com
PPT The Atom and The Periodic Table PowerPoint Presentation, free What Is The Atomic Number For Z The atomic number is the number of protons found in the nucleus of an atom, which uniquely identifies its element. An atom can be classified as a particular. This means that the number of protons is the characteristic which makes each element unique compared to all other elements. For example, the atomic number (z) for sodium (na) is 11. The. What Is The Atomic Number For Z.
From studylib.net
Atomic Number What Is The Atomic Number For Z The atomic number is the number of protons found in the nucleus of an atom, which uniquely identifies its element. Prior to 1915, the symbol z denoted the position. Elements are different because of their atomic number. This means that the number of protons is the characteristic which makes each element unique compared to all other elements. An atom can. What Is The Atomic Number For Z.
From studylib.net
Atomic Number (Z) What Is The Atomic Number For Z This means that the number of protons is the characteristic which makes each element unique compared to all other elements. The symbol for atomic number, z, stands for zahl, which means number in german. The symbol for the atomic number is designated with the letter z. The atomic number is the number of protons found in the nucleus of an. What Is The Atomic Number For Z.
From www.youtube.com
What is Atomic Number? Examples Chemistry YouTube What Is The Atomic Number For Z The atomic number is also called the proton number. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. Elements are different because of their atomic number. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element.. What Is The Atomic Number For Z.
From byjus.com
What is the graphical representation of energy of electron and atomic What Is The Atomic Number For Z For example, the atomic number (z) for sodium (na) is 11. Elements are different because of their atomic number. The atomic number is the number of protons found in the nucleus of an atom, which uniquely identifies its element. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. The atomic number. What Is The Atomic Number For Z.
From byjus.com
consider an atom with atomic number z as consisting of a positive point What Is The Atomic Number For Z The atomic number is the number of protons found in the nucleus of an atom, which uniquely identifies its element. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element. This means that the number of protons is the characteristic which makes each element unique. What Is The Atomic Number For Z.
From sciencenotes.org
What Is an Atomic Number? Definition and Examples What Is The Atomic Number For Z The symbol for the atomic number is designated with the letter z. The symbol for atomic number, z, stands for zahl, which means number in german. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element. Prior to 1915, the symbol z denoted the position.. What Is The Atomic Number For Z.
From www.slideserve.com
PPT Chapter 2 PowerPoint Presentation, free download ID6993806 What Is The Atomic Number For Z An atom can be classified as a particular. That means that all sodium atoms have 11 protons. For example, the atomic number (z) for sodium (na) is 11. The atomic number is also called the proton number. Prior to 1915, the symbol z denoted the position. 119 rows here is a list of elements of the periodic table, their atomic. What Is The Atomic Number For Z.
From www.slideserve.com
PPT “ Atomic Structure” PowerPoint Presentation, free download ID What Is The Atomic Number For Z 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element. An atom can be classified as a particular. The atomic number is also called the proton number.. What Is The Atomic Number For Z.
From studylib.net
X A Z Nuclear Notation What Is The Atomic Number For Z An atom can be classified as a particular. That means that all sodium atoms have 11 protons. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element. The symbol for atomic number, z, stands for zahl, which means number in german. This means that the. What Is The Atomic Number For Z.
From www.slideserve.com
PPT AP Chapter 2 PowerPoint Presentation, free download ID4057745 What Is The Atomic Number For Z The atomic number is also called the proton number. An atom can be classified as a particular. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. The atomic number is the number of protons found in the nucleus of an atom, which uniquely identifies its element. This means that the number. What Is The Atomic Number For Z.