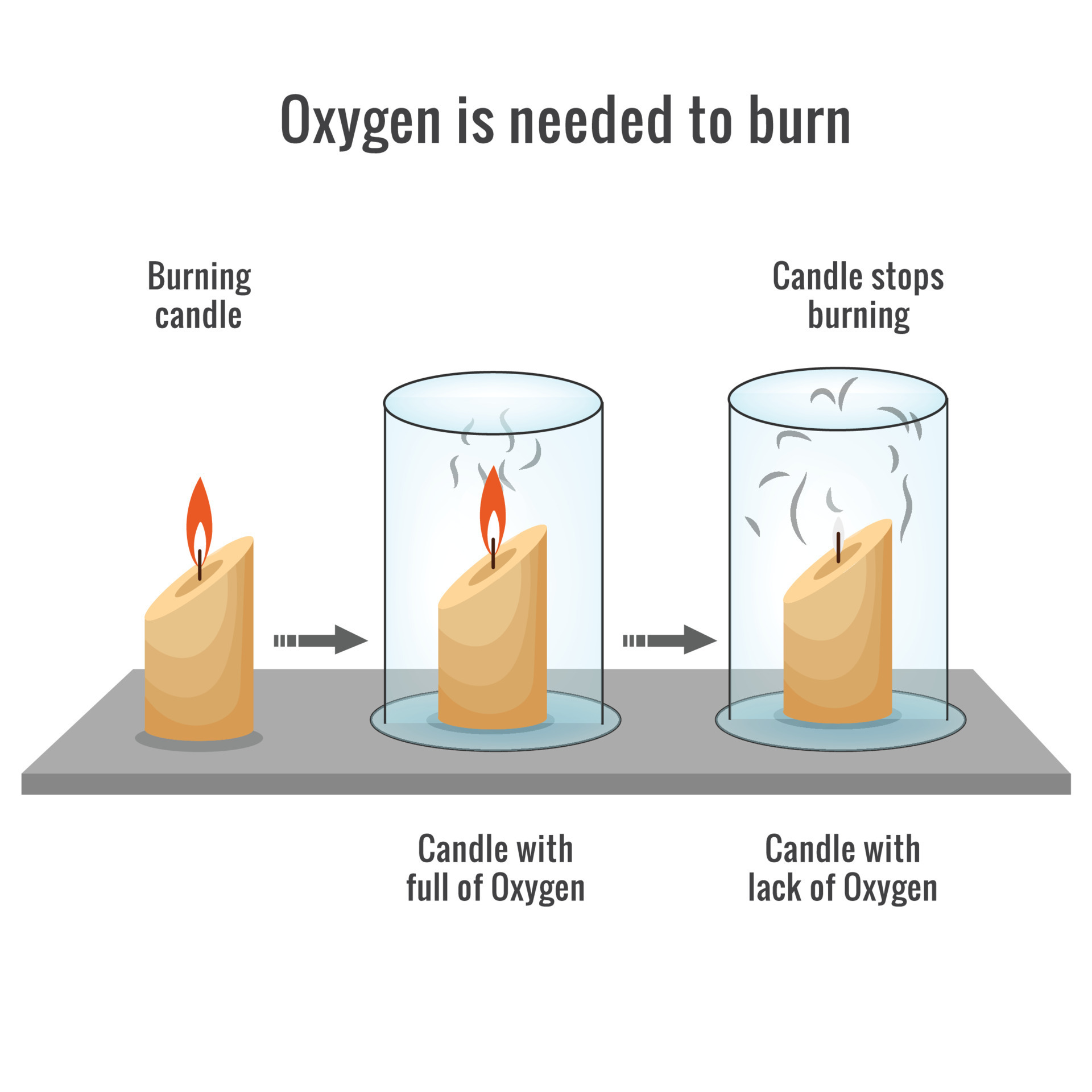
Candle Burning In Pure Oxygen . Some examples of element combustion are shown below. Josh finds out that in larger. An oxide is a compound containing both the element and oxygen chemically combined together. In zero gravity there is no ‘up’ and a flame forms a sphere. This makes the flame go out. In general, however, when a pure element burns in oxygen the product is called an oxide. This process creates co2 which is a colorless gas. Wax is derived from petroleum and is a carbon chemical and it reacts with the oxygen present in the air. The heat of the flame produces an updraught of air that draws the flame into its familiar shape. A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. During a combustion reaction, oxygen reacts chemically with the substance being burned. A combustion reaction is commonly referred to as “burning”. Next time you blow out a candle,. When you trap the candle in a jar, it only has a limited amount of oxygen. When oxygen is pushed away from the wick, it can’t react with the wax anymore.
from www.vecteezy.com
Next time you blow out a candle,. A combustion reaction is commonly referred to as “burning”. The heat of the flame produces an updraught of air that draws the flame into its familiar shape. When you trap the candle in a jar, it only has a limited amount of oxygen. Combustion is the chemical reaction happening in the candle burning process. This makes the flame go out. An oxide is a compound containing both the element and oxygen chemically combined together. Josh finds out that in larger. In general, however, when a pure element burns in oxygen the product is called an oxide. In zero gravity there is no ‘up’ and a flame forms a sphere.
Oxygen is needed for burning a candle 23452917 Vector Art at Vecteezy
Candle Burning In Pure Oxygen The heat of the flame produces an updraught of air that draws the flame into its familiar shape. Combustion is the chemical reaction happening in the candle burning process. During a combustion reaction, oxygen reacts chemically with the substance being burned. A combustion reaction is commonly referred to as “burning”. Josh finds out that in larger. When oxygen is pushed away from the wick, it can’t react with the wax anymore. Some examples of element combustion are shown below. In zero gravity there is no ‘up’ and a flame forms a sphere. When you trap the candle in a jar, it only has a limited amount of oxygen. This makes the flame go out. The heat of the flame produces an updraught of air that draws the flame into its familiar shape. Nitrogen slows down the burning process so you get enough energy through the day, bit by bit. In general, however, when a pure element burns in oxygen the product is called an oxide. A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. Next time you blow out a candle,. This process creates co2 which is a colorless gas.
From flamestuff.com
Do Candles Use Up Oxygen? Candle Burning In Pure Oxygen A combustion reaction is commonly referred to as “burning”. Some examples of element combustion are shown below. Next time you blow out a candle,. The heat of the flame produces an updraught of air that draws the flame into its familiar shape. When oxygen is pushed away from the wick, it can’t react with the wax anymore. In general, however,. Candle Burning In Pure Oxygen.
From melscience.com
Burning in pure oxygen MEL Chemistry Candle Burning In Pure Oxygen Next time you blow out a candle,. An oxide is a compound containing both the element and oxygen chemically combined together. A combustion reaction is commonly referred to as “burning”. Nitrogen slows down the burning process so you get enough energy through the day, bit by bit. If you breathed pure oxygen, the energy from your food would be released. Candle Burning In Pure Oxygen.
From www.dreamstime.com
Candle uses Oxygen 2 stock photo. Image of school, candle 101375580 Candle Burning In Pure Oxygen This process creates co2 which is a colorless gas. If you breathed pure oxygen, the energy from your food would be released all at once. In general, however, when a pure element burns in oxygen the product is called an oxide. When you trap the candle in a jar, it only has a limited amount of oxygen. An oxide is. Candle Burning In Pure Oxygen.
From www.youtube.com
Candles Need Oxygen YouTube Candle Burning In Pure Oxygen The heat of the flame produces an updraught of air that draws the flame into its familiar shape. A combustion reaction is commonly referred to as “burning”. If you breathed pure oxygen, the energy from your food would be released all at once. An oxide is a compound containing both the element and oxygen chemically combined together. When you trap. Candle Burning In Pure Oxygen.
From mekelas.blogspot.com
Combustion Experiment With Candle / function Oxygen In the Combustion Candle Burning In Pure Oxygen The heat of the flame produces an updraught of air that draws the flame into its familiar shape. Wax is derived from petroleum and is a carbon chemical and it reacts with the oxygen present in the air. This makes the flame go out. During a combustion reaction, oxygen reacts chemically with the substance being burned. When you trap the. Candle Burning In Pure Oxygen.
From www.britannica.com
How a Candle Flame Burns Britannica Candle Burning In Pure Oxygen If you breathed pure oxygen, the energy from your food would be released all at once. An oxide is a compound containing both the element and oxygen chemically combined together. Josh finds out that in larger. Some examples of element combustion are shown below. When oxygen is pushed away from the wick, it can’t react with the wax anymore. The. Candle Burning In Pure Oxygen.
From fphoto.photoshelter.com
science chemistry oxidation reaction candle burning oxygen Candle Burning In Pure Oxygen This process creates co2 which is a colorless gas. Wax is derived from petroleum and is a carbon chemical and it reacts with the oxygen present in the air. An oxide is a compound containing both the element and oxygen chemically combined together. When you trap the candle in a jar, it only has a limited amount of oxygen. This. Candle Burning In Pure Oxygen.
From pubs.sciepub.com
Figure 2. A candle is burning Oxygen Gives Life; Oxygen Takes Away Candle Burning In Pure Oxygen If you breathed pure oxygen, the energy from your food would be released all at once. When oxygen is pushed away from the wick, it can’t react with the wax anymore. When you trap the candle in a jar, it only has a limited amount of oxygen. An oxide is a compound containing both the element and oxygen chemically combined. Candle Burning In Pure Oxygen.
From www.alamy.com
Oxygen candles Stock Videos & Footage HD and 4K Video Clips Alamy Candle Burning In Pure Oxygen This process creates co2 which is a colorless gas. A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. Combustion is the chemical reaction happening in the candle burning process. Next time you blow out a candle,. The heat of the flame produces an updraught of air that draws the flame into. Candle Burning In Pure Oxygen.
From cleaningexec.com
Major Causes Of House Fires And What You Can Do To Prevent Them Candle Burning In Pure Oxygen In general, however, when a pure element burns in oxygen the product is called an oxide. A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. An oxide is a compound containing both the element and oxygen chemically combined together. Wax is derived from petroleum and is a carbon chemical and it. Candle Burning In Pure Oxygen.
From www.researchgate.net
Procedure of candleburning experiment. (a) Place the sealed Candle Burning In Pure Oxygen The heat of the flame produces an updraught of air that draws the flame into its familiar shape. If you breathed pure oxygen, the energy from your food would be released all at once. A combustion reaction is commonly referred to as “burning”. An oxide is a compound containing both the element and oxygen chemically combined together. Wax is derived. Candle Burning In Pure Oxygen.
From www.shutterstock.com
2,725 Candle experiment Images, Stock Photos & Vectors Shutterstock Candle Burning In Pure Oxygen This process creates co2 which is a colorless gas. When oxygen is pushed away from the wick, it can’t react with the wax anymore. Josh finds out that in larger. Nitrogen slows down the burning process so you get enough energy through the day, bit by bit. During a combustion reaction, oxygen reacts chemically with the substance being burned. The. Candle Burning In Pure Oxygen.
From www.alamy.com
Oxygen needed for fire and burning. vector diagram to demonstrate the Candle Burning In Pure Oxygen This makes the flame go out. Some examples of element combustion are shown below. Josh finds out that in larger. Next time you blow out a candle,. Nitrogen slows down the burning process so you get enough energy through the day, bit by bit. A combustion reaction is commonly referred to as “burning”. The heat of the flame produces an. Candle Burning In Pure Oxygen.
From www.glam.com
How To Keep Your Favorite Candles Burning Evenly Candle Burning In Pure Oxygen Josh finds out that in larger. An oxide is a compound containing both the element and oxygen chemically combined together. This process creates co2 which is a colorless gas. The heat of the flame produces an updraught of air that draws the flame into its familiar shape. Nitrogen slows down the burning process so you get enough energy through the. Candle Burning In Pure Oxygen.
From www.youtube.com
Vacuum Candle Experiment YouTube Candle Burning In Pure Oxygen When oxygen is pushed away from the wick, it can’t react with the wax anymore. Combustion is the chemical reaction happening in the candle burning process. An oxide is a compound containing both the element and oxygen chemically combined together. In general, however, when a pure element burns in oxygen the product is called an oxide. Nitrogen slows down the. Candle Burning In Pure Oxygen.
From www.youtube.com
How does oxygen affect burning candles? YouTube Candle Burning In Pure Oxygen Wax is derived from petroleum and is a carbon chemical and it reacts with the oxygen present in the air. Nitrogen slows down the burning process so you get enough energy through the day, bit by bit. Josh finds out that in larger. Some examples of element combustion are shown below. When oxygen is pushed away from the wick, it. Candle Burning In Pure Oxygen.
From fphoto.photoshelter.com
science chemistry oxidation reaction candle burning oxygen Candle Burning In Pure Oxygen When oxygen is pushed away from the wick, it can’t react with the wax anymore. During a combustion reaction, oxygen reacts chemically with the substance being burned. If you breathed pure oxygen, the energy from your food would be released all at once. Combustion is the chemical reaction happening in the candle burning process. The heat of the flame produces. Candle Burning In Pure Oxygen.
From www.alamy.com
Sodium burning in pure oxygen, image 1 of 2. This is an exothermic Candle Burning In Pure Oxygen Next time you blow out a candle,. This makes the flame go out. Combustion is the chemical reaction happening in the candle burning process. An oxide is a compound containing both the element and oxygen chemically combined together. If you breathed pure oxygen, the energy from your food would be released all at once. When you trap the candle in. Candle Burning In Pure Oxygen.
From fphoto.photoshelter.com
science chemistry oxidation reaction candle burning oxygen Candle Burning In Pure Oxygen During a combustion reaction, oxygen reacts chemically with the substance being burned. This makes the flame go out. A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. Josh finds out that in larger. When you trap the candle in a jar, it only has a limited amount of oxygen. In zero. Candle Burning In Pure Oxygen.
From www.dreamstime.com
Oxygen Candle Photos Free & RoyaltyFree Stock Photos from Dreamstime Candle Burning In Pure Oxygen Wax is derived from petroleum and is a carbon chemical and it reacts with the oxygen present in the air. The heat of the flame produces an updraught of air that draws the flame into its familiar shape. A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. Some examples of element. Candle Burning In Pure Oxygen.
From unbelievable-facts.com
What is an Oxygen Candle? Candle Burning In Pure Oxygen A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. An oxide is a compound containing both the element and oxygen chemically combined together. Some examples of element combustion are shown below. Josh finds out that in larger. When oxygen is pushed away from the wick, it can’t react with the wax. Candle Burning In Pure Oxygen.
From www.dreamstime.com
Candle uses Oxygen 1 stock photo. Image of laboratory 101376582 Candle Burning In Pure Oxygen A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. An oxide is a compound containing both the element and oxygen chemically combined together. Josh finds out that in larger. When oxygen is pushed away from the wick, it can’t react with the wax anymore. In zero gravity there is no ‘up’. Candle Burning In Pure Oxygen.
From scholarsark.com
What Form Of Energy Is Transformed In A Burning Candle? Scholars Ark Candle Burning In Pure Oxygen In general, however, when a pure element burns in oxygen the product is called an oxide. A combustion reaction is commonly referred to as “burning”. This makes the flame go out. A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. When oxygen is pushed away from the wick, it can’t react. Candle Burning In Pure Oxygen.
From www.alamy.com
fire and oxygen. Science experiment with two candles and glass. Burning Candle Burning In Pure Oxygen A combustion reaction is commonly referred to as “burning”. This makes the flame go out. Next time you blow out a candle,. In zero gravity there is no ‘up’ and a flame forms a sphere. When oxygen is pushed away from the wick, it can’t react with the wax anymore. If you breathed pure oxygen, the energy from your food. Candle Burning In Pure Oxygen.
From www.youtube.com
Air Is Needed For Burning Activity/Experiment For Kids At Home YouTube Candle Burning In Pure Oxygen During a combustion reaction, oxygen reacts chemically with the substance being burned. Combustion is the chemical reaction happening in the candle burning process. An oxide is a compound containing both the element and oxygen chemically combined together. Wax is derived from petroleum and is a carbon chemical and it reacts with the oxygen present in the air. Josh finds out. Candle Burning In Pure Oxygen.
From mekelas.blogspot.com
Combustion Experiment With Candle / function Oxygen In the Combustion Candle Burning In Pure Oxygen Wax is derived from petroleum and is a carbon chemical and it reacts with the oxygen present in the air. In general, however, when a pure element burns in oxygen the product is called an oxide. Combustion is the chemical reaction happening in the candle burning process. When you trap the candle in a jar, it only has a limited. Candle Burning In Pure Oxygen.
From www.shutterstock.com
Oxygen Fire Burning Experiment Closing Glass Stock Photo 2227522133 Candle Burning In Pure Oxygen This process creates co2 which is a colorless gas. In zero gravity there is no ‘up’ and a flame forms a sphere. If you breathed pure oxygen, the energy from your food would be released all at once. Nitrogen slows down the burning process so you get enough energy through the day, bit by bit. A candle flame is the. Candle Burning In Pure Oxygen.
From www.dreamstime.com
Fire and Oxygen. Experiment with Two Candles and Glass. Burning and Candle Burning In Pure Oxygen In zero gravity there is no ‘up’ and a flame forms a sphere. This makes the flame go out. When you trap the candle in a jar, it only has a limited amount of oxygen. Some examples of element combustion are shown below. An oxide is a compound containing both the element and oxygen chemically combined together. A candle flame. Candle Burning In Pure Oxygen.
From www.dreamstime.com
Etup that Shows How Candles Use Oxygen. Stock Photo Image of hand Candle Burning In Pure Oxygen Nitrogen slows down the burning process so you get enough energy through the day, bit by bit. This makes the flame go out. A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. Josh finds out that in larger. Wax is derived from petroleum and is a carbon chemical and it reacts. Candle Burning In Pure Oxygen.
From www.youtube.com
Oxygen is necessary for burning candle इनमें से कौन सा candle पहले Candle Burning In Pure Oxygen Wax is derived from petroleum and is a carbon chemical and it reacts with the oxygen present in the air. When oxygen is pushed away from the wick, it can’t react with the wax anymore. Next time you blow out a candle,. A candle flame is the result of a chemical reaction between wax gas and oxygen in the air.. Candle Burning In Pure Oxygen.
From www.vecteezy.com
Oxygen is needed for burning a candle 23452917 Vector Art at Vecteezy Candle Burning In Pure Oxygen Wax is derived from petroleum and is a carbon chemical and it reacts with the oxygen present in the air. When you trap the candle in a jar, it only has a limited amount of oxygen. Next time you blow out a candle,. Some examples of element combustion are shown below. During a combustion reaction, oxygen reacts chemically with the. Candle Burning In Pure Oxygen.
From commons.wikimedia.org
FileCandle burning.jpg Candle Burning In Pure Oxygen If you breathed pure oxygen, the energy from your food would be released all at once. When oxygen is pushed away from the wick, it can’t react with the wax anymore. In general, however, when a pure element burns in oxygen the product is called an oxide. In zero gravity there is no ‘up’ and a flame forms a sphere.. Candle Burning In Pure Oxygen.
From www.youtube.com
Burning Candle Full HD YouTube Candle Burning In Pure Oxygen Next time you blow out a candle,. When oxygen is pushed away from the wick, it can’t react with the wax anymore. This process creates co2 which is a colorless gas. During a combustion reaction, oxygen reacts chemically with the substance being burned. A candle flame is the result of a chemical reaction between wax gas and oxygen in the. Candle Burning In Pure Oxygen.
From www.istockphoto.com
Oxygen Fire And Combustion Vacuum Candle Burning Experiment Isolated On Candle Burning In Pure Oxygen Josh finds out that in larger. The heat of the flame produces an updraught of air that draws the flame into its familiar shape. A combustion reaction is commonly referred to as “burning”. Next time you blow out a candle,. If you breathed pure oxygen, the energy from your food would be released all at once. Combustion is the chemical. Candle Burning In Pure Oxygen.
From www.youtube.com
Candle oxygen experiment YouTube Candle Burning In Pure Oxygen In zero gravity there is no ‘up’ and a flame forms a sphere. When you trap the candle in a jar, it only has a limited amount of oxygen. Next time you blow out a candle,. A combustion reaction is commonly referred to as “burning”. This makes the flame go out. During a combustion reaction, oxygen reacts chemically with the. Candle Burning In Pure Oxygen.