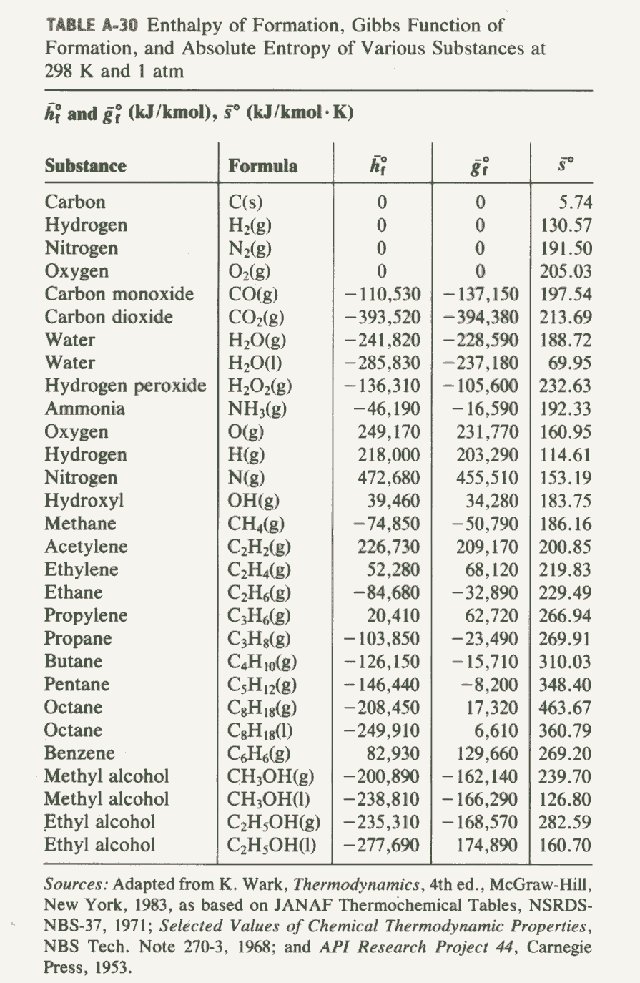
Standard Heat Of Formation For H2O . The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. Mallard, eds, nist chemistry webbook, nist standard reference database. This is the enthalpy change for the exothermic reaction: It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of. All standard state, 25 °c and 1 bar (written to 1 decimal place). Similarly, hydrogen is h 2 (g), not atomic hydrogen (h).
from rayb78.github.io
A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. Similarly, hydrogen is h 2 (g), not atomic hydrogen (h). Mallard, eds, nist chemistry webbook, nist standard reference database. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. All standard state, 25 °c and 1 bar (written to 1 decimal place). This is the enthalpy change for the exothermic reaction:
Heat Of Formation Chart
Standard Heat Of Formation For H2O Mallard, eds, nist chemistry webbook, nist standard reference database. Similarly, hydrogen is h 2 (g), not atomic hydrogen (h). The standard enthalpy of formation of any element in its standard state is zero by definition. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of. The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. This is the enthalpy change for the exothermic reaction: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. Mallard, eds, nist chemistry webbook, nist standard reference database. All standard state, 25 °c and 1 bar (written to 1 decimal place). For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c.
From slideplayer.com
Chapter 10 “Thermochemistry” ppt download Standard Heat Of Formation For H2O Mallard, eds, nist chemistry webbook, nist standard reference database. All standard state, 25 °c and 1 bar (written to 1 decimal place). Similarly, hydrogen is h 2 (g), not atomic hydrogen (h). A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. This is. Standard Heat Of Formation For H2O.
From www.toppr.com
+ Test Summary Q 77/200 If standard enthalpy of formation of CO2 (g Standard Heat Of Formation For H2O Similarly, hydrogen is h 2 (g), not atomic hydrogen (h). For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. This is the enthalpy change for the exothermic reaction: A standard enthalpy of formation δ h f ° δ. Standard Heat Of Formation For H2O.
From www.youtube.com
DAT Finding Standard Heat of Formation C(s) + H2O(g) → CO(g) + H2(g Standard Heat Of Formation For H2O The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. All standard state, 25 °c and 1 bar (written to 1 decimal place). Similarly, hydrogen is h 2 (g), not atomic hydrogen (h). The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. It. Standard Heat Of Formation For H2O.
From www.slideserve.com
PPT Chemistry 17.4 PowerPoint Presentation, free download ID2772524 Standard Heat Of Formation For H2O The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole. Standard Heat Of Formation For H2O.
From www.numerade.com
Consider the following reaction 2 H2S (g) + 3 O2 (g) → 2 SO2 (g) + 2 Standard Heat Of Formation For H2O All standard state, 25 °c and 1 bar (written to 1 decimal place). For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. The standard enthalpy of formation of any element in its standard state is zero by definition.. Standard Heat Of Formation For H2O.
From slideplayer.com
Calculating Heats of Reaction ppt download Standard Heat Of Formation For H2O For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. The standard enthalpy. Standard Heat Of Formation For H2O.
From www.slideserve.com
PPT Hess’s Law PowerPoint Presentation, free download ID6793985 Standard Heat Of Formation For H2O For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of. Mallard, eds,. Standard Heat Of Formation For H2O.
From www.chegg.com
Solved The standard enthalpy of formation of H2O(g) at 298 K Standard Heat Of Formation For H2O This is the enthalpy change for the exothermic reaction: It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard.. Standard Heat Of Formation For H2O.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation and the standard Standard Heat Of Formation For H2O For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. This is the enthalpy change for the exothermic reaction: A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction. Standard Heat Of Formation For H2O.
From byjus.com
The standard molar heat for formation ofethane, carbondioxide and water Standard Heat Of Formation For H2O It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of. All standard state, 25 °c and 1 bar (written to 1 decimal place). This is the enthalpy change for the exothermic reaction: For example, although oxygen can exist as ozone (o 3), atomic. Standard Heat Of Formation For H2O.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Standard Heat Of Formation For H2O Similarly, hydrogen is h 2 (g), not atomic hydrogen (h). For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. All standard state, 25 °c and 1 bar (written to 1 decimal place). This is the enthalpy change for. Standard Heat Of Formation For H2O.
From quizzlistreplevies.z13.web.core.windows.net
How To Calculate The Heat Of Formation Standard Heat Of Formation For H2O Similarly, hydrogen is h 2 (g), not atomic hydrogen (h). For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. It means that 33.2 kj of energy. Standard Heat Of Formation For H2O.
From www.youtube.com
Change in enthalpy for reaction, 2H2O2(l)—2H2O(l)+O2(g) if heat of Standard Heat Of Formation For H2O The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change. Standard Heat Of Formation For H2O.
From mavink.com
Standard Enthalpy Chart Standard Heat Of Formation For H2O This is the enthalpy change for the exothermic reaction: The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. For example, although oxygen can exist as ozone (o. Standard Heat Of Formation For H2O.
From studylib.net
heats of formation worksheet key Standard Heat Of Formation For H2O For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and. Standard Heat Of Formation For H2O.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Heat Of Formation For H2O It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of. Similarly, hydrogen is h 2 (g), not atomic hydrogen (h). The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. A standard enthalpy of formation δ h f ° δ h. Standard Heat Of Formation For H2O.
From www.toppr.com
The standard enthalpy of formation of H2O (l) and Fe2O3 (s) are Standard Heat Of Formation For H2O The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under. Standard Heat Of Formation For H2O.
From brainly.com
The standard enthalpy of formation of h2o Standard Heat Of Formation For H2O 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. This is the enthalpy change for the exothermic reaction: It means that 33.2. Standard Heat Of Formation For H2O.
From www.slideserve.com
PPT Energy Transformations PowerPoint Presentation, free download Standard Heat Of Formation For H2O All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly. Standard Heat Of Formation For H2O.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Heat Of Formation For H2O The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. Mallard, eds, nist chemistry webbook, nist standard reference database. Similarly, hydrogen is h 2 (g), not atomic hydrogen (h). For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure. Standard Heat Of Formation For H2O.
From hydrogengasgaosube.blogspot.com
Hydrogen Gas June 2017 Standard Heat Of Formation For H2O The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2. Standard Heat Of Formation For H2O.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Formation for Standard Heat Of Formation For H2O It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of. Standard Heat Of Formation For H2O.
From www.toppr.com
The standard heat of formation at 298 K for CCl4(g),H2O(g),CO2(g),HCl(g Standard Heat Of Formation For H2O This is the enthalpy change for the exothermic reaction: The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol.. Standard Heat Of Formation For H2O.
From edurev.in
Standard heat of formation of CH4, CO2 and H2O are 76.2, 394.8, 241. Standard Heat Of Formation For H2O 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of. The standard enthalpy of the formation of nitrogen dioxide is. Standard Heat Of Formation For H2O.
From ar.inspiredpencil.com
Heat Of Combustion Table Kjmol Standard Heat Of Formation For H2O The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. All standard state, 25 °c and 1 bar (written to 1 decimal place). This is the enthalpy change for the exothermic reaction:. Standard Heat Of Formation For H2O.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download Standard Heat Of Formation For H2O The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of.. Standard Heat Of Formation For H2O.
From www.slideserve.com
PPT CHAPTER 4 PowerPoint Presentation ID3180471 Standard Heat Of Formation For H2O Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most. Standard Heat Of Formation For H2O.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download Standard Heat Of Formation For H2O The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation δ h f ° δ h f ° is an. Standard Heat Of Formation For H2O.
From slideplayer.com
Chemistry ppt download Standard Heat Of Formation For H2O The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. Mallard, eds, nist chemistry webbook, nist standard reference database. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. The standard enthalpy of formation of any element. Standard Heat Of Formation For H2O.
From www.toppr.com
Heat of formation of H2O is 188 kJ/mol and H2O2 is 286 kJ/mol. The Standard Heat Of Formation For H2O The standard enthalpy of formation of any element in its standard state is zero by definition. This is the enthalpy change for the exothermic reaction: It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of. Mallard, eds, nist chemistry webbook, nist standard reference. Standard Heat Of Formation For H2O.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Heat Of Formation For H2O 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. This is the enthalpy change for the exothermic reaction: The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy. Standard Heat Of Formation For H2O.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Heat Of Formation For H2O The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. Mallard, eds, nist chemistry webbook, nist standard reference database. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one. Standard Heat Of Formation For H2O.
From www.slideshare.net
Lect w10 abbrev_ thermochemistry_alg Standard Heat Of Formation For H2O This is the enthalpy change for the exothermic reaction: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. 193 rows in. Standard Heat Of Formation For H2O.
From rayb78.github.io
Heat Of Formation Chart Standard Heat Of Formation For H2O 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. All standard state, 25 °c and 1 bar (written to 1 decimal place). For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable. Standard Heat Of Formation For H2O.
From readingandwritingprojectcom.web.fc2.com
heat of formation for o2 Standard Heat Of Formation For H2O The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. Similarly, hydrogen is h 2 (g), not atomic hydrogen (h). For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. Mallard, eds, nist chemistry webbook, nist standard. Standard Heat Of Formation For H2O.