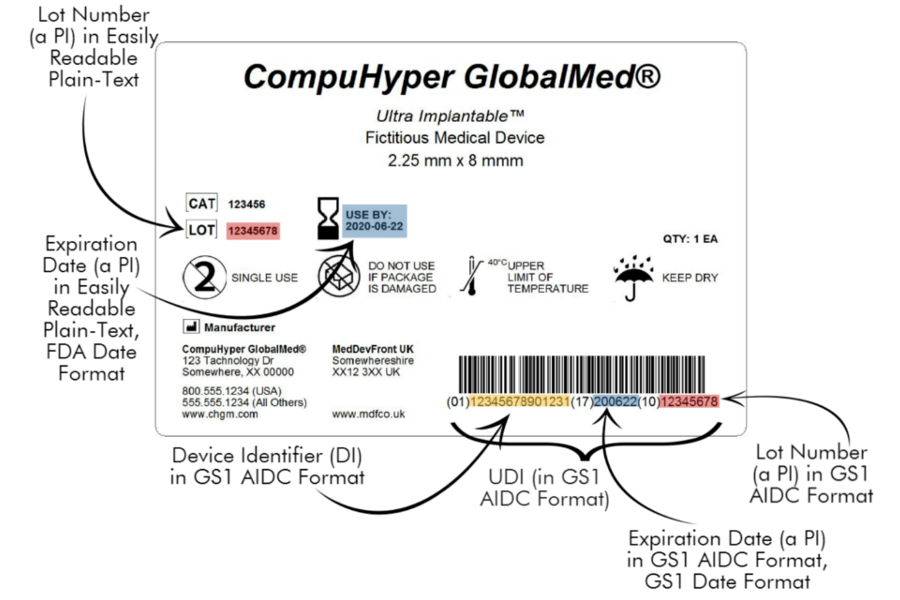
Tga Medical Device Labelling Requirements . For active implantable medical devices, the device. All medical devices in australia must meet safety and performance. Guidance on tgo 91 and tgo 92. All product labelling must be in english. Required advisory statements for medicine labels (rasml) advisory statements apply to all. Labelling means the labels and information that come with a medical device. Information for manufacturers and sponsors on meeting medical device labelling requirements. At least one category (or more if it applies) must be selected for each ivd. Information about any risks associated with its implantation. The name the ivd must be as presented on the product labelling. Essential principle 13 of schedule 1 of the therapeutic goods (medical devices) regulations 2002 (the regulations) and regulation 10.2. In summary, the tga explains that the labeling requirements for medical devices, as outlined by the therapeutic goods (medical.
from hiveta.com
For active implantable medical devices, the device. The name the ivd must be as presented on the product labelling. Required advisory statements for medicine labels (rasml) advisory statements apply to all. Information about any risks associated with its implantation. Essential principle 13 of schedule 1 of the therapeutic goods (medical devices) regulations 2002 (the regulations) and regulation 10.2. All medical devices in australia must meet safety and performance. At least one category (or more if it applies) must be selected for each ivd. Guidance on tgo 91 and tgo 92. All product labelling must be in english. Labelling means the labels and information that come with a medical device.
Label Compliance AB&R® (American Barcode and RFID)
Tga Medical Device Labelling Requirements Information for manufacturers and sponsors on meeting medical device labelling requirements. Essential principle 13 of schedule 1 of the therapeutic goods (medical devices) regulations 2002 (the regulations) and regulation 10.2. At least one category (or more if it applies) must be selected for each ivd. All medical devices in australia must meet safety and performance. Required advisory statements for medicine labels (rasml) advisory statements apply to all. Guidance on tgo 91 and tgo 92. Information about any risks associated with its implantation. In summary, the tga explains that the labeling requirements for medical devices, as outlined by the therapeutic goods (medical. The name the ivd must be as presented on the product labelling. Information for manufacturers and sponsors on meeting medical device labelling requirements. All product labelling must be in english. For active implantable medical devices, the device. Labelling means the labels and information that come with a medical device.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Tga Medical Device Labelling Requirements For active implantable medical devices, the device. At least one category (or more if it applies) must be selected for each ivd. All medical devices in australia must meet safety and performance. Essential principle 13 of schedule 1 of the therapeutic goods (medical devices) regulations 2002 (the regulations) and regulation 10.2. Information for manufacturers and sponsors on meeting medical device. Tga Medical Device Labelling Requirements.
From old.sermitsiaq.ag
Medical Device Label Template Tga Medical Device Labelling Requirements Labelling means the labels and information that come with a medical device. Essential principle 13 of schedule 1 of the therapeutic goods (medical devices) regulations 2002 (the regulations) and regulation 10.2. All medical devices in australia must meet safety and performance. Information about any risks associated with its implantation. Guidance on tgo 91 and tgo 92. All product labelling must. Tga Medical Device Labelling Requirements.
From platohealth.ai
Ultimate Guide To Device Class Requirements Under EU MDR PlatoHealth Tga Medical Device Labelling Requirements Essential principle 13 of schedule 1 of the therapeutic goods (medical devices) regulations 2002 (the regulations) and regulation 10.2. All product labelling must be in english. All medical devices in australia must meet safety and performance. Guidance on tgo 91 and tgo 92. In summary, the tga explains that the labeling requirements for medical devices, as outlined by the therapeutic. Tga Medical Device Labelling Requirements.
From exodjaqsq.blob.core.windows.net
Tga Medical Device Labeling Requirements at Tyrone Gaylord blog Tga Medical Device Labelling Requirements All product labelling must be in english. Information for manufacturers and sponsors on meeting medical device labelling requirements. For active implantable medical devices, the device. In summary, the tga explains that the labeling requirements for medical devices, as outlined by the therapeutic goods (medical. Labelling means the labels and information that come with a medical device. Essential principle 13 of. Tga Medical Device Labelling Requirements.
From www.regulatoryaffairsnews.com
TGA MD Guidance Medical Device Labelling Obligations Tga Medical Device Labelling Requirements The name the ivd must be as presented on the product labelling. Essential principle 13 of schedule 1 of the therapeutic goods (medical devices) regulations 2002 (the regulations) and regulation 10.2. Labelling means the labels and information that come with a medical device. Information for manufacturers and sponsors on meeting medical device labelling requirements. In summary, the tga explains that. Tga Medical Device Labelling Requirements.
From exodjaqsq.blob.core.windows.net
Tga Medical Device Labeling Requirements at Tyrone Gaylord blog Tga Medical Device Labelling Requirements All medical devices in australia must meet safety and performance. The name the ivd must be as presented on the product labelling. Guidance on tgo 91 and tgo 92. At least one category (or more if it applies) must be selected for each ivd. Information for manufacturers and sponsors on meeting medical device labelling requirements. Labelling means the labels and. Tga Medical Device Labelling Requirements.
From www.schlafenderhase.com
Medical Device Labeling Requirements Schlafender Hase Tga Medical Device Labelling Requirements Information about any risks associated with its implantation. The name the ivd must be as presented on the product labelling. Guidance on tgo 91 and tgo 92. Essential principle 13 of schedule 1 of the therapeutic goods (medical devices) regulations 2002 (the regulations) and regulation 10.2. All medical devices in australia must meet safety and performance. In summary, the tga. Tga Medical Device Labelling Requirements.
From fyojaorux.blob.core.windows.net
Medical Device Regulations Part 3 at Willie Dunford blog Tga Medical Device Labelling Requirements All product labelling must be in english. Information about any risks associated with its implantation. Information for manufacturers and sponsors on meeting medical device labelling requirements. Essential principle 13 of schedule 1 of the therapeutic goods (medical devices) regulations 2002 (the regulations) and regulation 10.2. Required advisory statements for medicine labels (rasml) advisory statements apply to all. The name the. Tga Medical Device Labelling Requirements.
From old.sermitsiaq.ag
Medical Device Label Template Tga Medical Device Labelling Requirements Information about any risks associated with its implantation. Essential principle 13 of schedule 1 of the therapeutic goods (medical devices) regulations 2002 (the regulations) and regulation 10.2. Guidance on tgo 91 and tgo 92. Information for manufacturers and sponsors on meeting medical device labelling requirements. All product labelling must be in english. For active implantable medical devices, the device. At. Tga Medical Device Labelling Requirements.
From exogphupj.blob.core.windows.net
Medical Device Labelling Tga at William Maurer blog Tga Medical Device Labelling Requirements Guidance on tgo 91 and tgo 92. For active implantable medical devices, the device. Essential principle 13 of schedule 1 of the therapeutic goods (medical devices) regulations 2002 (the regulations) and regulation 10.2. Labelling means the labels and information that come with a medical device. Required advisory statements for medicine labels (rasml) advisory statements apply to all. Information for manufacturers. Tga Medical Device Labelling Requirements.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Tga Medical Device Labelling Requirements Information for manufacturers and sponsors on meeting medical device labelling requirements. The name the ivd must be as presented on the product labelling. At least one category (or more if it applies) must be selected for each ivd. All product labelling must be in english. In summary, the tga explains that the labeling requirements for medical devices, as outlined by. Tga Medical Device Labelling Requirements.
From www.vrogue.co
Canadian Device Labeling Requirements Ce Mark Package vrogue.co Tga Medical Device Labelling Requirements The name the ivd must be as presented on the product labelling. Guidance on tgo 91 and tgo 92. Essential principle 13 of schedule 1 of the therapeutic goods (medical devices) regulations 2002 (the regulations) and regulation 10.2. All product labelling must be in english. In summary, the tga explains that the labeling requirements for medical devices, as outlined by. Tga Medical Device Labelling Requirements.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Tga Medical Device Labelling Requirements For active implantable medical devices, the device. All product labelling must be in english. In summary, the tga explains that the labeling requirements for medical devices, as outlined by the therapeutic goods (medical. Labelling means the labels and information that come with a medical device. Information for manufacturers and sponsors on meeting medical device labelling requirements. Required advisory statements for. Tga Medical Device Labelling Requirements.
From exodjaqsq.blob.core.windows.net
Tga Medical Device Labeling Requirements at Tyrone Gaylord blog Tga Medical Device Labelling Requirements All medical devices in australia must meet safety and performance. Required advisory statements for medicine labels (rasml) advisory statements apply to all. Labelling means the labels and information that come with a medical device. For active implantable medical devices, the device. Essential principle 13 of schedule 1 of the therapeutic goods (medical devices) regulations 2002 (the regulations) and regulation 10.2.. Tga Medical Device Labelling Requirements.
From www.vrogue.co
Symbols Commonly Used In Medical Device Packaging And vrogue.co Tga Medical Device Labelling Requirements Information about any risks associated with its implantation. Required advisory statements for medicine labels (rasml) advisory statements apply to all. At least one category (or more if it applies) must be selected for each ivd. Labelling means the labels and information that come with a medical device. Essential principle 13 of schedule 1 of the therapeutic goods (medical devices) regulations. Tga Medical Device Labelling Requirements.
From hxeskyyxe.blob.core.windows.net
Fda Medical Device Label Requirements at James Day blog Tga Medical Device Labelling Requirements At least one category (or more if it applies) must be selected for each ivd. All medical devices in australia must meet safety and performance. In summary, the tga explains that the labeling requirements for medical devices, as outlined by the therapeutic goods (medical. The name the ivd must be as presented on the product labelling. Guidance on tgo 91. Tga Medical Device Labelling Requirements.
From angelanjohnson.com
Medical Devices Angela N Johnson Tga Medical Device Labelling Requirements Required advisory statements for medicine labels (rasml) advisory statements apply to all. Labelling means the labels and information that come with a medical device. All product labelling must be in english. At least one category (or more if it applies) must be selected for each ivd. All medical devices in australia must meet safety and performance. In summary, the tga. Tga Medical Device Labelling Requirements.
From mavink.com
Medical Device Labeling Symbols Tga Medical Device Labelling Requirements Labelling means the labels and information that come with a medical device. The name the ivd must be as presented on the product labelling. Information about any risks associated with its implantation. For active implantable medical devices, the device. In summary, the tga explains that the labeling requirements for medical devices, as outlined by the therapeutic goods (medical. At least. Tga Medical Device Labelling Requirements.
From hxeskyyxe.blob.core.windows.net
Fda Medical Device Label Requirements at James Day blog Tga Medical Device Labelling Requirements The name the ivd must be as presented on the product labelling. Labelling means the labels and information that come with a medical device. For active implantable medical devices, the device. Information about any risks associated with its implantation. At least one category (or more if it applies) must be selected for each ivd. Information for manufacturers and sponsors on. Tga Medical Device Labelling Requirements.
From www.regdesk.co
TGA on Changes to Implementation Delay RegDesk Tga Medical Device Labelling Requirements Information about any risks associated with its implantation. Guidance on tgo 91 and tgo 92. All product labelling must be in english. At least one category (or more if it applies) must be selected for each ivd. Required advisory statements for medicine labels (rasml) advisory statements apply to all. Labelling means the labels and information that come with a medical. Tga Medical Device Labelling Requirements.
From clin-r.com
Labels for Medical Devices Clin R Tga Medical Device Labelling Requirements Information for manufacturers and sponsors on meeting medical device labelling requirements. Required advisory statements for medicine labels (rasml) advisory statements apply to all. For active implantable medical devices, the device. Information about any risks associated with its implantation. In summary, the tga explains that the labeling requirements for medical devices, as outlined by the therapeutic goods (medical. Essential principle 13. Tga Medical Device Labelling Requirements.
From www.pdffiller.com
Fillable Online Comment Request; Medical Device Labeling Regulations Tga Medical Device Labelling Requirements In summary, the tga explains that the labeling requirements for medical devices, as outlined by the therapeutic goods (medical. Guidance on tgo 91 and tgo 92. Labelling means the labels and information that come with a medical device. All product labelling must be in english. Information for manufacturers and sponsors on meeting medical device labelling requirements. All medical devices in. Tga Medical Device Labelling Requirements.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Tga Medical Device Labelling Requirements Labelling means the labels and information that come with a medical device. The name the ivd must be as presented on the product labelling. In summary, the tga explains that the labeling requirements for medical devices, as outlined by the therapeutic goods (medical. Required advisory statements for medicine labels (rasml) advisory statements apply to all. Information about any risks associated. Tga Medical Device Labelling Requirements.
From exodjaqsq.blob.core.windows.net
Tga Medical Device Labeling Requirements at Tyrone Gaylord blog Tga Medical Device Labelling Requirements Required advisory statements for medicine labels (rasml) advisory statements apply to all. All medical devices in australia must meet safety and performance. Information for manufacturers and sponsors on meeting medical device labelling requirements. For active implantable medical devices, the device. Essential principle 13 of schedule 1 of the therapeutic goods (medical devices) regulations 2002 (the regulations) and regulation 10.2. In. Tga Medical Device Labelling Requirements.
From www.tapecon.com
What Information Should You Include on Your Medical Device Label? Tga Medical Device Labelling Requirements Information about any risks associated with its implantation. The name the ivd must be as presented on the product labelling. At least one category (or more if it applies) must be selected for each ivd. Labelling means the labels and information that come with a medical device. For active implantable medical devices, the device. In summary, the tga explains that. Tga Medical Device Labelling Requirements.
From clin-r.com
Labels for Medical Devices Clin R Tga Medical Device Labelling Requirements Guidance on tgo 91 and tgo 92. At least one category (or more if it applies) must be selected for each ivd. Information for manufacturers and sponsors on meeting medical device labelling requirements. The name the ivd must be as presented on the product labelling. For active implantable medical devices, the device. All product labelling must be in english. Essential. Tga Medical Device Labelling Requirements.
From www.greenlight.guru
Am I Complying with FDA Medical Device Labeling Requirements? Tga Medical Device Labelling Requirements Essential principle 13 of schedule 1 of the therapeutic goods (medical devices) regulations 2002 (the regulations) and regulation 10.2. Guidance on tgo 91 and tgo 92. For active implantable medical devices, the device. Information about any risks associated with its implantation. All product labelling must be in english. At least one category (or more if it applies) must be selected. Tga Medical Device Labelling Requirements.
From exogphupj.blob.core.windows.net
Medical Device Labelling Tga at William Maurer blog Tga Medical Device Labelling Requirements All product labelling must be in english. Information about any risks associated with its implantation. For active implantable medical devices, the device. At least one category (or more if it applies) must be selected for each ivd. In summary, the tga explains that the labeling requirements for medical devices, as outlined by the therapeutic goods (medical. Labelling means the labels. Tga Medical Device Labelling Requirements.
From www.regdesk.co
HSA Guidance on Labeling for Medical Devices Introduction RegDesk Tga Medical Device Labelling Requirements Labelling means the labels and information that come with a medical device. Required advisory statements for medicine labels (rasml) advisory statements apply to all. The name the ivd must be as presented on the product labelling. At least one category (or more if it applies) must be selected for each ivd. Information for manufacturers and sponsors on meeting medical device. Tga Medical Device Labelling Requirements.
From blog.clevercompliance.io
EU Medical Device Labelling Requirements Clever Compliance Tga Medical Device Labelling Requirements Required advisory statements for medicine labels (rasml) advisory statements apply to all. All product labelling must be in english. Labelling means the labels and information that come with a medical device. All medical devices in australia must meet safety and performance. Guidance on tgo 91 and tgo 92. In summary, the tga explains that the labeling requirements for medical devices,. Tga Medical Device Labelling Requirements.
From exodjaqsq.blob.core.windows.net
Tga Medical Device Labeling Requirements at Tyrone Gaylord blog Tga Medical Device Labelling Requirements Labelling means the labels and information that come with a medical device. All product labelling must be in english. Guidance on tgo 91 and tgo 92. For active implantable medical devices, the device. Required advisory statements for medicine labels (rasml) advisory statements apply to all. All medical devices in australia must meet safety and performance. Information about any risks associated. Tga Medical Device Labelling Requirements.
From hiveta.com
Label Compliance AB&R® (American Barcode and RFID) Tga Medical Device Labelling Requirements All product labelling must be in english. Essential principle 13 of schedule 1 of the therapeutic goods (medical devices) regulations 2002 (the regulations) and regulation 10.2. For active implantable medical devices, the device. Required advisory statements for medicine labels (rasml) advisory statements apply to all. In summary, the tga explains that the labeling requirements for medical devices, as outlined by. Tga Medical Device Labelling Requirements.
From exovighly.blob.core.windows.net
Fda Medical Device Private Label Distributor at Jerry Robertson blog Tga Medical Device Labelling Requirements All product labelling must be in english. Information about any risks associated with its implantation. At least one category (or more if it applies) must be selected for each ivd. Labelling means the labels and information that come with a medical device. Guidance on tgo 91 and tgo 92. Essential principle 13 of schedule 1 of the therapeutic goods (medical. Tga Medical Device Labelling Requirements.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Tga Medical Device Labelling Requirements At least one category (or more if it applies) must be selected for each ivd. Labelling means the labels and information that come with a medical device. Required advisory statements for medicine labels (rasml) advisory statements apply to all. For active implantable medical devices, the device. The name the ivd must be as presented on the product labelling. Essential principle. Tga Medical Device Labelling Requirements.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Tga Medical Device Labelling Requirements Labelling means the labels and information that come with a medical device. All product labelling must be in english. The name the ivd must be as presented on the product labelling. Information about any risks associated with its implantation. At least one category (or more if it applies) must be selected for each ivd. In summary, the tga explains that. Tga Medical Device Labelling Requirements.