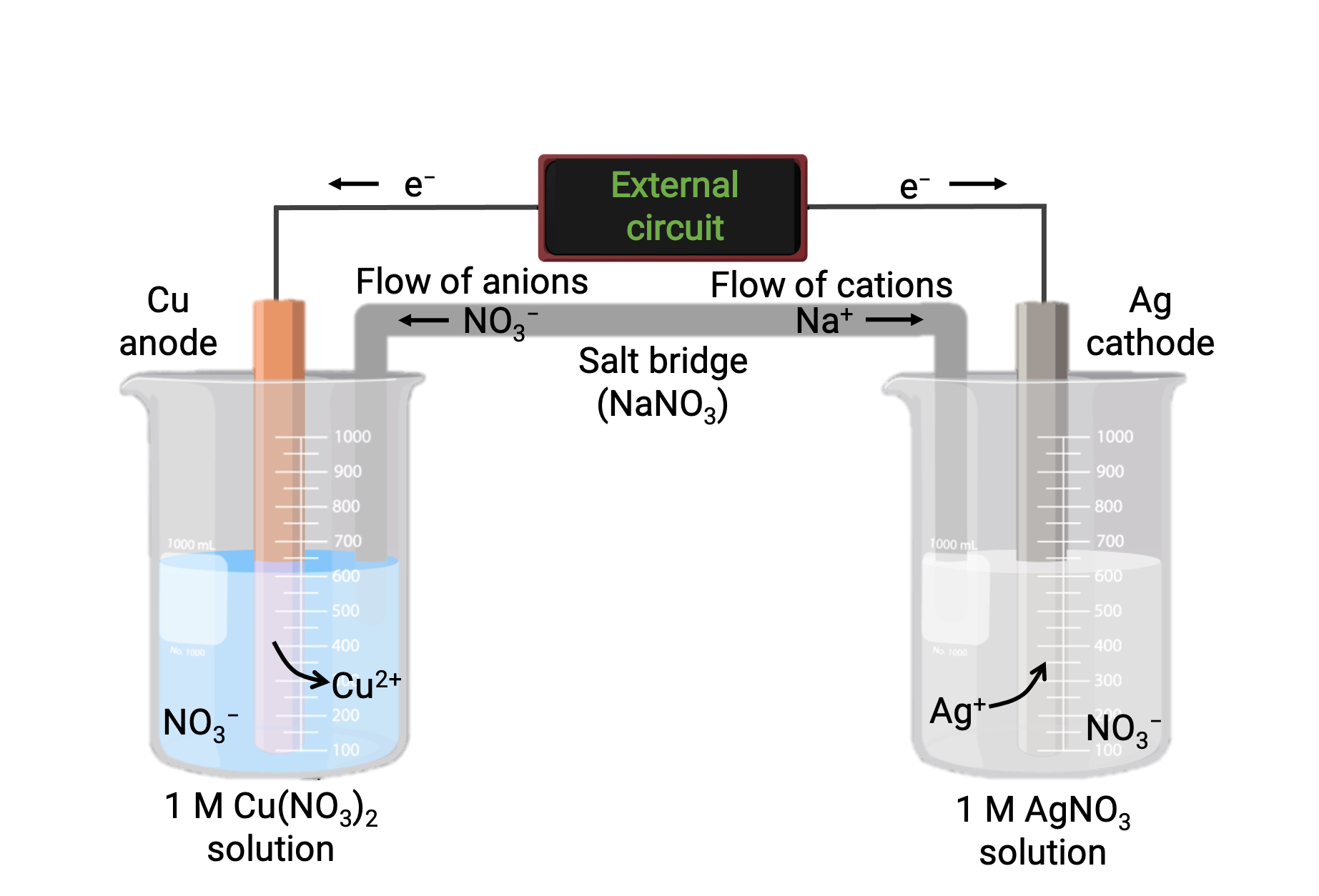
Galvanic Ionization . Cohesive (“lattice”) energies and ionization energies in water. There are four elements necessary for corrosion to occur in a galvanic cell: Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions:
from www.jove.com
Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. Cohesive (“lattice”) energies and ionization energies in water. In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions: There are four elements necessary for corrosion to occur in a galvanic cell:
Voltaic/Galvanic Cells Principle, Components, Cell Notation JoVE
Galvanic Ionization There are four elements necessary for corrosion to occur in a galvanic cell: In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions: There are four elements necessary for corrosion to occur in a galvanic cell: Cohesive (“lattice”) energies and ionization energies in water. Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path.
From ar.inspiredpencil.com
Galvanic Cell Galvanic Ionization Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. There are four elements necessary for corrosion to occur in a galvanic cell: Cohesive (“lattice”) energies and ionization energies in water. In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in. Galvanic Ionization.
From ar.inspiredpencil.com
Galvanic Cell Equation Galvanic Ionization Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. Cohesive (“lattice”) energies and ionization energies in water. There are four elements necessary for corrosion to occur in a galvanic cell: In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in. Galvanic Ionization.
From chem.libretexts.org
1 Electrochemical Cells (Experiment) Chemistry LibreTexts Galvanic Ionization Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. There are four elements necessary for corrosion to occur in a galvanic cell: Cohesive (“lattice”) energies and ionization energies in water. In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in. Galvanic Ionization.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps Galvanic Ionization There are four elements necessary for corrosion to occur in a galvanic cell: Cohesive (“lattice”) energies and ionization energies in water. In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions: Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an. Galvanic Ionization.
From chem.libretexts.org
19.6 Commercial Galvanic Cells Chemistry LibreTexts Galvanic Ionization There are four elements necessary for corrosion to occur in a galvanic cell: Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. Cohesive (“lattice”) energies and ionization energies in water. In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in. Galvanic Ionization.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID9592789 Galvanic Ionization Cohesive (“lattice”) energies and ionization energies in water. Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. There are four elements necessary for corrosion to occur in a galvanic cell: In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in. Galvanic Ionization.
From www.dynamicscience.com.au
chemistry galvanic cells Galvanic Ionization Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions: Cohesive (“lattice”) energies and ionization energies in water. There are four elements necessary for corrosion. Galvanic Ionization.
From www.researchgate.net
Atomic view of the BiZn galvanic displacement in an acidic medium Galvanic Ionization Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. There are four elements necessary for corrosion to occur in a galvanic cell: Cohesive (“lattice”) energies and ionization energies in water. In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in. Galvanic Ionization.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Galvanic Ionization There are four elements necessary for corrosion to occur in a galvanic cell: In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions: Cohesive (“lattice”) energies and ionization energies in water. Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an. Galvanic Ionization.
From www.geeksforgeeks.org
Galvanic Cell Definition, Construction, Working Principle Galvanic Ionization In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions: Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. There are four elements necessary for corrosion to occur in a galvanic cell: Cohesive (“lattice”). Galvanic Ionization.
From scienceinfo.com
Galvanic Cell (Voltaic Cell) Definition, Working Principle Galvanic Ionization Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. Cohesive (“lattice”) energies and ionization energies in water. There are four elements necessary for corrosion to occur in a galvanic cell: In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in. Galvanic Ionization.
From chem.libretexts.org
Electrolysis I Chemistry LibreTexts Galvanic Ionization Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions: There are four elements necessary for corrosion to occur in a galvanic cell: Cohesive (“lattice”). Galvanic Ionization.
From psu.pb.unizin.org
Galvanic Cells (17.2) Chemistry 112 (Chapters 1217 of OpenStax Galvanic Ionization Cohesive (“lattice”) energies and ionization energies in water. In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions: Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. There are four elements necessary for corrosion. Galvanic Ionization.
From www.vectorstock.com
Electrolysis process galvanic cell element Vector Image Galvanic Ionization Cohesive (“lattice”) energies and ionization energies in water. There are four elements necessary for corrosion to occur in a galvanic cell: Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in. Galvanic Ionization.
From glossary.periodni.com
Galvanic Chemistry Dictionary & Glossary Galvanic Ionization There are four elements necessary for corrosion to occur in a galvanic cell: In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions: Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. Cohesive (“lattice”). Galvanic Ionization.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID5404902 Galvanic Ionization There are four elements necessary for corrosion to occur in a galvanic cell: Cohesive (“lattice”) energies and ionization energies in water. In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions: Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an. Galvanic Ionization.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps Galvanic Ionization In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions: Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. Cohesive (“lattice”) energies and ionization energies in water. There are four elements necessary for corrosion. Galvanic Ionization.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Galvanic Ionization There are four elements necessary for corrosion to occur in a galvanic cell: Cohesive (“lattice”) energies and ionization energies in water. In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions: Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an. Galvanic Ionization.
From www.youtube.com
Calculations involving Galvanic Cells YouTube Galvanic Ionization Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. There are four elements necessary for corrosion to occur in a galvanic cell: In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions: Cohesive (“lattice”). Galvanic Ionization.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation JoVE Galvanic Ionization Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. Cohesive (“lattice”) energies and ionization energies in water. There are four elements necessary for corrosion to occur in a galvanic cell: In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in. Galvanic Ionization.
From quizlet.com
GALVANIC CELL Diagram Quizlet Galvanic Ionization Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. There are four elements necessary for corrosion to occur in a galvanic cell: Cohesive (“lattice”) energies and ionization energies in water. In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in. Galvanic Ionization.
From www.scienceabc.com
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC Galvanic Ionization Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. There are four elements necessary for corrosion to occur in a galvanic cell: Cohesive (“lattice”) energies and ionization energies in water. In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in. Galvanic Ionization.
From www.horiba.com
Electrochemical Cell Method Galvanic Cell Type HORIBA Galvanic Ionization Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. Cohesive (“lattice”) energies and ionization energies in water. There are four elements necessary for corrosion to occur in a galvanic cell: In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in. Galvanic Ionization.
From courses.lumenlearning.com
Galvanic Cells Chemistry for Majors Galvanic Ionization Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. Cohesive (“lattice”) energies and ionization energies in water. In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions: There are four elements necessary for corrosion. Galvanic Ionization.
From edurev.in
Daniel Cell Or Galvanic Cell Class 12 Notes EduRev Galvanic Ionization There are four elements necessary for corrosion to occur in a galvanic cell: Cohesive (“lattice”) energies and ionization energies in water. Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in. Galvanic Ionization.
From chem.libretexts.org
11.1 Galvanic Cells Chemistry LibreTexts Galvanic Ionization In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions: There are four elements necessary for corrosion to occur in a galvanic cell: Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. Cohesive (“lattice”). Galvanic Ionization.
From slideplayer.com
Electricity. ppt download Galvanic Ionization Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. There are four elements necessary for corrosion to occur in a galvanic cell: Cohesive (“lattice”) energies and ionization energies in water. In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in. Galvanic Ionization.
From www.researchgate.net
Schematic of corrosion processes and mechanism (a) the galvanic couple Galvanic Ionization In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions: There are four elements necessary for corrosion to occur in a galvanic cell: Cohesive (“lattice”) energies and ionization energies in water. Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an. Galvanic Ionization.
From www.pinterest.com
7 Segment LED Display Tutorial For Dummies Electrochemistry, Galvanic Galvanic Ionization There are four elements necessary for corrosion to occur in a galvanic cell: In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions: Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. Cohesive (“lattice”). Galvanic Ionization.
From stock.adobe.com
Vettoriale Stock Voltaic galvanic cell or daniell cell.Redox reaction Galvanic Ionization Cohesive (“lattice”) energies and ionization energies in water. There are four elements necessary for corrosion to occur in a galvanic cell: Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in. Galvanic Ionization.
From chem.libretexts.org
2.1 Galvanic Cells Chemistry LibreTexts Galvanic Ionization Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. There are four elements necessary for corrosion to occur in a galvanic cell: In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions: Cohesive (“lattice”). Galvanic Ionization.
From www.pinterest.com
Galvanic Cell Animation Galvanic cell, Cell, Electrical energy Galvanic Ionization Cohesive (“lattice”) energies and ionization energies in water. In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions: There are four elements necessary for corrosion to occur in a galvanic cell: Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an. Galvanic Ionization.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps Galvanic Ionization Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions: There are four elements necessary for corrosion to occur in a galvanic cell: Cohesive (“lattice”). Galvanic Ionization.
From chemistrytalk.org
Galvanic Cells & Voltaic Cells Electrochemical Cells ChemTalk Galvanic Ionization In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions: Cohesive (“lattice”) energies and ionization energies in water. There are four elements necessary for corrosion to occur in a galvanic cell: Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an. Galvanic Ionization.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Galvanic Ionization Cohesive (“lattice”) energies and ionization energies in water. Galvanic corrosion is an electrochemical action of two dissimilar metals in the presence of an electrolyte and an electron conductive path. In this paper, a simple explanation of the energy of simple batteries or galvanic cells is given in terms of two conceptually meaningful contributions: There are four elements necessary for corrosion. Galvanic Ionization.