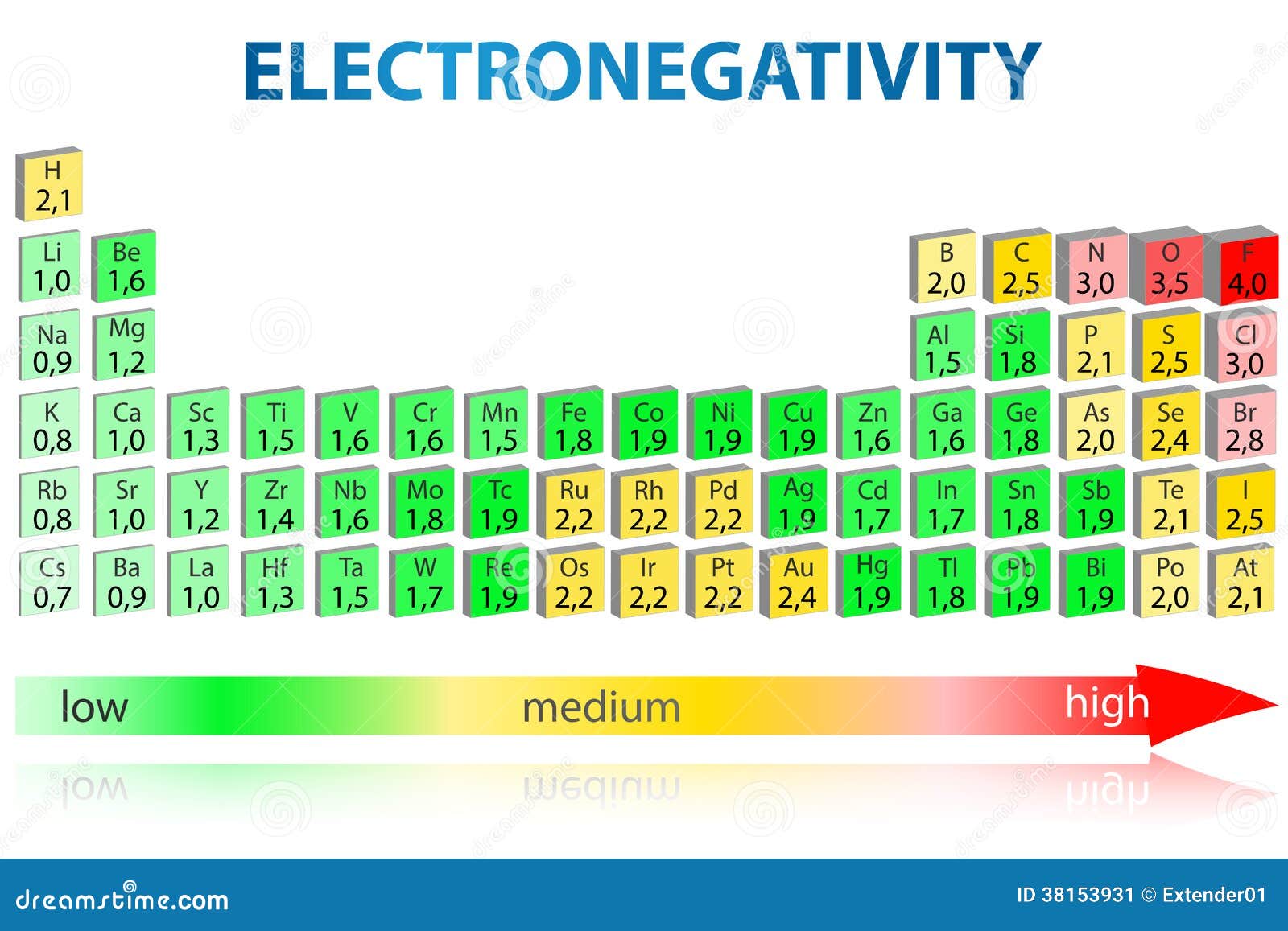
Tin Electronegativity . Tin has an electronegativity of 1.8 (pauling original) or 1.96 (pauling) according to different scales. The suggested values are all taken from. The electronegativity of tin is: Learn about the different types and values of electronegativity for tin, a chemical element with symbol sn and atomic number 50. Electronegativity is a chemical property that describes how well an atom can attract an electron to itself. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. Pauling’s electronegativity scale has a fundamental value and uses accessible thermochemical data, but fails at predicting the. Almost all elements with electronegativity below 3 are metals; Find more information about tin's. In general, an atom’s electronegativity is affected by both its atomic number and the. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. The few exceptions are si (2.82), ge (2.79), sn (2.68, but sn is known at normal.
from www.dreamstime.com
Pauling’s electronegativity scale has a fundamental value and uses accessible thermochemical data, but fails at predicting the. The few exceptions are si (2.82), ge (2.79), sn (2.68, but sn is known at normal. Find more information about tin's. The electronegativity of tin is: The suggested values are all taken from. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. In general, an atom’s electronegativity is affected by both its atomic number and the. Electronegativity is a chemical property that describes how well an atom can attract an electron to itself. Tin has an electronegativity of 1.8 (pauling original) or 1.96 (pauling) according to different scales. 103 rows electronegativity is not a uniquely defined property and may depend on the definition.
Electronegativity Periodic Table Stock Illustration Illustration of
Tin Electronegativity Electronegativity is a chemical property that describes how well an atom can attract an electron to itself. The suggested values are all taken from. Almost all elements with electronegativity below 3 are metals; 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Find more information about tin's. Tin has an electronegativity of 1.8 (pauling original) or 1.96 (pauling) according to different scales. In general, an atom’s electronegativity is affected by both its atomic number and the. The electronegativity of tin is: 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. Electronegativity is a chemical property that describes how well an atom can attract an electron to itself. Pauling’s electronegativity scale has a fundamental value and uses accessible thermochemical data, but fails at predicting the. The few exceptions are si (2.82), ge (2.79), sn (2.68, but sn is known at normal. Learn about the different types and values of electronegativity for tin, a chemical element with symbol sn and atomic number 50.
From sciencenotes.org
List of Electronegativity Values of the Elements Tin Electronegativity 103 rows electronegativity is not a uniquely defined property and may depend on the definition. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. The electronegativity of tin is: The few exceptions are si (2.82), ge (2.79), sn (2.68, but sn is known at normal. In general, an atom’s electronegativity is affected. Tin Electronegativity.
From chemistry.com.pk
Electronegativity and Electronegativity Chart in PDF Tin Electronegativity 103 rows electronegativity is not a uniquely defined property and may depend on the definition. In general, an atom’s electronegativity is affected by both its atomic number and the. The few exceptions are si (2.82), ge (2.79), sn (2.68, but sn is known at normal. 119 rows find the electronegativity values of all the elements in a table, from hydrogen. Tin Electronegativity.
From www.dreamstime.com
Tin Chemical Element with First Ionization Energy, Atomic Mass and Tin Electronegativity Pauling’s electronegativity scale has a fundamental value and uses accessible thermochemical data, but fails at predicting the. The electronegativity of tin is: Tin has an electronegativity of 1.8 (pauling original) or 1.96 (pauling) according to different scales. Electronegativity is a chemical property that describes how well an atom can attract an electron to itself. The suggested values are all taken. Tin Electronegativity.
From general.chemistrysteps.com
Electronegativity and Bond Polarity Chemistry Steps Tin Electronegativity Tin has an electronegativity of 1.8 (pauling original) or 1.96 (pauling) according to different scales. The electronegativity of tin is: The few exceptions are si (2.82), ge (2.79), sn (2.68, but sn is known at normal. Learn about the different types and values of electronegativity for tin, a chemical element with symbol sn and atomic number 50. Pauling’s electronegativity scale. Tin Electronegativity.
From www.alamy.com
Tin chemical element with first ionization energy, atomic mass and Tin Electronegativity The electronegativity of tin is: Learn about the different types and values of electronegativity for tin, a chemical element with symbol sn and atomic number 50. Tin has an electronegativity of 1.8 (pauling original) or 1.96 (pauling) according to different scales. The few exceptions are si (2.82), ge (2.79), sn (2.68, but sn is known at normal. Find more information. Tin Electronegativity.
From chemistry.com.pk
Electronegativity and Electronegativity Chart in PDF Tin Electronegativity The electronegativity of tin is: Pauling’s electronegativity scale has a fundamental value and uses accessible thermochemical data, but fails at predicting the. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. The suggested values are all taken from. The few exceptions are si (2.82), ge (2.79), sn (2.68, but sn is known. Tin Electronegativity.
From www.sliderbase.com
Periodic Table of Electronegativities Tin Electronegativity 103 rows electronegativity is not a uniquely defined property and may depend on the definition. The electronegativity of tin is: Tin has an electronegativity of 1.8 (pauling original) or 1.96 (pauling) according to different scales. The suggested values are all taken from. Pauling’s electronegativity scale has a fundamental value and uses accessible thermochemical data, but fails at predicting the. Almost. Tin Electronegativity.
From www.numerade.com
SOLVEDPlace the following elements in order of increasing Tin Electronegativity The suggested values are all taken from. Pauling’s electronegativity scale has a fundamental value and uses accessible thermochemical data, but fails at predicting the. In general, an atom’s electronegativity is affected by both its atomic number and the. Almost all elements with electronegativity below 3 are metals; The electronegativity of tin is: Electronegativity is a chemical property that describes how. Tin Electronegativity.
From www.nuclear-power.com
Tin Electron Affinity Electronegativity Ionization Energy of Tin Tin Electronegativity The few exceptions are si (2.82), ge (2.79), sn (2.68, but sn is known at normal. Pauling’s electronegativity scale has a fundamental value and uses accessible thermochemical data, but fails at predicting the. Find more information about tin's. The suggested values are all taken from. The electronegativity of tin is: Almost all elements with electronegativity below 3 are metals; In. Tin Electronegativity.
From mavink.com
Printable Electronegativity Table Tin Electronegativity Electronegativity is a chemical property that describes how well an atom can attract an electron to itself. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. The few exceptions are si (2.82), ge (2.79), sn (2.68, but. Tin Electronegativity.
From electraschematics.com
Understanding the Tin Orbital Diagram A Complete Guide for Beginners Tin Electronegativity Find more information about tin's. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. In general, an atom’s electronegativity is affected by both its atomic number and the. The electronegativity of tin is: The suggested values are all taken from. 103 rows electronegativity is not a uniquely defined property and may depend. Tin Electronegativity.
From chem.libretexts.org
8.4 Bond Polarity and Electronegativity Chemistry LibreTexts Tin Electronegativity 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Almost all elements with electronegativity below 3 are metals; The suggested values are all taken from. Find more information about tin's. Pauling’s electronegativity scale has a fundamental value and uses accessible thermochemical data, but fails at predicting the. 119 rows find the electronegativity values of. Tin Electronegativity.
From socratic.org
How does electronegativity change across a period? Socratic Tin Electronegativity Find more information about tin's. The few exceptions are si (2.82), ge (2.79), sn (2.68, but sn is known at normal. The electronegativity of tin is: The suggested values are all taken from. In general, an atom’s electronegativity is affected by both its atomic number and the. 103 rows electronegativity is not a uniquely defined property and may depend on. Tin Electronegativity.
From eventthyme.net
How To Find Electrons On Periodic Table How To Do Thing Tin Electronegativity Learn about the different types and values of electronegativity for tin, a chemical element with symbol sn and atomic number 50. The electronegativity of tin is: Find more information about tin's. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. Electronegativity is a chemical property that describes how well an atom can. Tin Electronegativity.
From www.chemistrylearner.com
Tin Definition, Facts, Symbol, Discovery, Property, Uses Tin Electronegativity 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. In general, an atom’s electronegativity is affected by both its atomic number and the. Almost all elements with electronegativity below 3 are metals; The electronegativity of tin is: Tin has an electronegativity of 1.8 (pauling original) or 1.96 (pauling) according to different scales.. Tin Electronegativity.
From www.dreamstime.com
Electronegativity Periodic Table Stock Illustration Illustration of Tin Electronegativity The few exceptions are si (2.82), ge (2.79), sn (2.68, but sn is known at normal. Find more information about tin's. The suggested values are all taken from. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Tin has an electronegativity of 1.8 (pauling original) or 1.96 (pauling) according to different scales. Almost all. Tin Electronegativity.
From learnwithdrscott.com
Electronegativity Table Easy Hard Science Tin Electronegativity The few exceptions are si (2.82), ge (2.79), sn (2.68, but sn is known at normal. Learn about the different types and values of electronegativity for tin, a chemical element with symbol sn and atomic number 50. Electronegativity is a chemical property that describes how well an atom can attract an electron to itself. Tin has an electronegativity of 1.8. Tin Electronegativity.
From mungfali.com
Electronegativity Difference Chart Tin Electronegativity Learn about the different types and values of electronegativity for tin, a chemical element with symbol sn and atomic number 50. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. Pauling’s electronegativity scale has a fundamental value and uses accessible thermochemical data, but fails at predicting the. Find more information about tin's.. Tin Electronegativity.
From mungfali.com
Electronegativity Chart And Lewis Structures Tin Electronegativity The suggested values are all taken from. Pauling’s electronegativity scale has a fundamental value and uses accessible thermochemical data, but fails at predicting the. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Almost all elements with. Tin Electronegativity.
From iperiodictable.com
What is Electronegativity Chart List of Electronegativity [PDF] Tin Electronegativity Almost all elements with electronegativity below 3 are metals; Electronegativity is a chemical property that describes how well an atom can attract an electron to itself. Tin has an electronegativity of 1.8 (pauling original) or 1.96 (pauling) according to different scales. The suggested values are all taken from. 103 rows electronegativity is not a uniquely defined property and may depend. Tin Electronegativity.
From blog.sciencenet.cn
科学网—元素电负性(electronegativity of elements)的理论预估值 耿华运的博文 Tin Electronegativity Pauling’s electronegativity scale has a fundamental value and uses accessible thermochemical data, but fails at predicting the. The suggested values are all taken from. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. The few exceptions are. Tin Electronegativity.
From facts.net
12 Mindblowing Facts About Electronegativity Tin Electronegativity 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. Almost all elements with electronegativity below 3 are metals; Find more information about tin's. In general, an atom’s electronegativity is affected by both its atomic number and the. 103 rows electronegativity is not a uniquely defined property and may depend on the definition.. Tin Electronegativity.
From dona.tompkinscountystructuralracism.org
Electronegativity Values Chart Understanding The Chemistry Of Elements Tin Electronegativity 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. In general, an atom’s electronegativity is affected by both its atomic number and the. The few exceptions are si (2.82), ge (2.79), sn (2.68, but sn is known at normal. Learn about the different types and values of electronegativity for tin, a chemical. Tin Electronegativity.
From www.studocu.com
Electronegativitychart freeprintablepaper 1 2 3 4 5 6 7 8 9 10 11 Tin Electronegativity Tin has an electronegativity of 1.8 (pauling original) or 1.96 (pauling) according to different scales. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. Pauling’s electronegativity scale has a fundamental value and uses accessible thermochemical data, but fails at predicting the. The electronegativity of tin is: The few exceptions are si (2.82),. Tin Electronegativity.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Tin Electronegativity Electronegativity is a chemical property that describes how well an atom can attract an electron to itself. The suggested values are all taken from. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Almost all elements with electronegativity below 3 are metals; In general, an atom’s electronegativity is affected by both its atomic number. Tin Electronegativity.
From surfguppy.com
What is Electronegativity? Tin Electronegativity Find more information about tin's. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. The suggested values are all taken from. Pauling’s electronegativity scale has a fundamental value and uses accessible thermochemical data, but fails at predicting. Tin Electronegativity.
From www.geeksforgeeks.org
Electronegativity Definition, Meaning, Periodic Trends, Examples Tin Electronegativity In general, an atom’s electronegativity is affected by both its atomic number and the. The electronegativity of tin is: Almost all elements with electronegativity below 3 are metals; 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. The few exceptions are si (2.82), ge (2.79), sn (2.68, but sn is known at. Tin Electronegativity.
From www.thoughtco.com
Electronegativity Definition and Examples Tin Electronegativity Find more information about tin's. Tin has an electronegativity of 1.8 (pauling original) or 1.96 (pauling) according to different scales. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. Almost all elements with electronegativity below 3 are metals; 103 rows electronegativity is not a uniquely defined property and may depend on the. Tin Electronegativity.
From mungfali.com
Electronegativity Trend Explained Tin Electronegativity The suggested values are all taken from. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. Learn about the different types and values of electronegativity for tin, a chemical element with symbol sn and atomic number 50. Pauling’s electronegativity scale has a fundamental value and uses accessible thermochemical data, but fails at. Tin Electronegativity.
From www.semanticscholar.org
Figure 1 from Isomer Shift and Electronegativity in Compounds of Tin Tin Electronegativity Almost all elements with electronegativity below 3 are metals; 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Pauling’s electronegativity scale has a fundamental value and uses accessible thermochemical data, but fails at predicting the. The few exceptions are si (2.82), ge (2.79), sn (2.68, but sn is known at normal. The suggested values. Tin Electronegativity.
From www.formsbirds.com
Basic Electronegativity Chart Free Download Tin Electronegativity Pauling’s electronegativity scale has a fundamental value and uses accessible thermochemical data, but fails at predicting the. The suggested values are all taken from. The electronegativity of tin is: Electronegativity is a chemical property that describes how well an atom can attract an electron to itself. Almost all elements with electronegativity below 3 are metals; Tin has an electronegativity of. Tin Electronegativity.
From revisionscience.com
Electronegativity ALevel Chemistry Tin Electronegativity Find more information about tin's. Learn about the different types and values of electronegativity for tin, a chemical element with symbol sn and atomic number 50. The few exceptions are si (2.82), ge (2.79), sn (2.68, but sn is known at normal. Tin has an electronegativity of 1.8 (pauling original) or 1.96 (pauling) according to different scales. Electronegativity is a. Tin Electronegativity.
From becuo.com
Periodic Table With Pauling Electronegativity Images & Pictures Becuo Tin Electronegativity Find more information about tin's. In general, an atom’s electronegativity is affected by both its atomic number and the. Learn about the different types and values of electronegativity for tin, a chemical element with symbol sn and atomic number 50. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. The suggested values. Tin Electronegativity.
From slideplayer.com
HC CHEMISTRY HC CHEMISTRY (B) Periodicity Bonding Continuum. ppt download Tin Electronegativity Pauling’s electronegativity scale has a fundamental value and uses accessible thermochemical data, but fails at predicting the. Tin has an electronegativity of 1.8 (pauling original) or 1.96 (pauling) according to different scales. The suggested values are all taken from. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Almost all elements with electronegativity below. Tin Electronegativity.
From knordslearning.com
Electronegativity Chart of all Elements (With Periodic table) Tin Electronegativity The few exceptions are si (2.82), ge (2.79), sn (2.68, but sn is known at normal. Tin has an electronegativity of 1.8 (pauling original) or 1.96 (pauling) according to different scales. Almost all elements with electronegativity below 3 are metals; The electronegativity of tin is: Electronegativity is a chemical property that describes how well an atom can attract an electron. Tin Electronegativity.