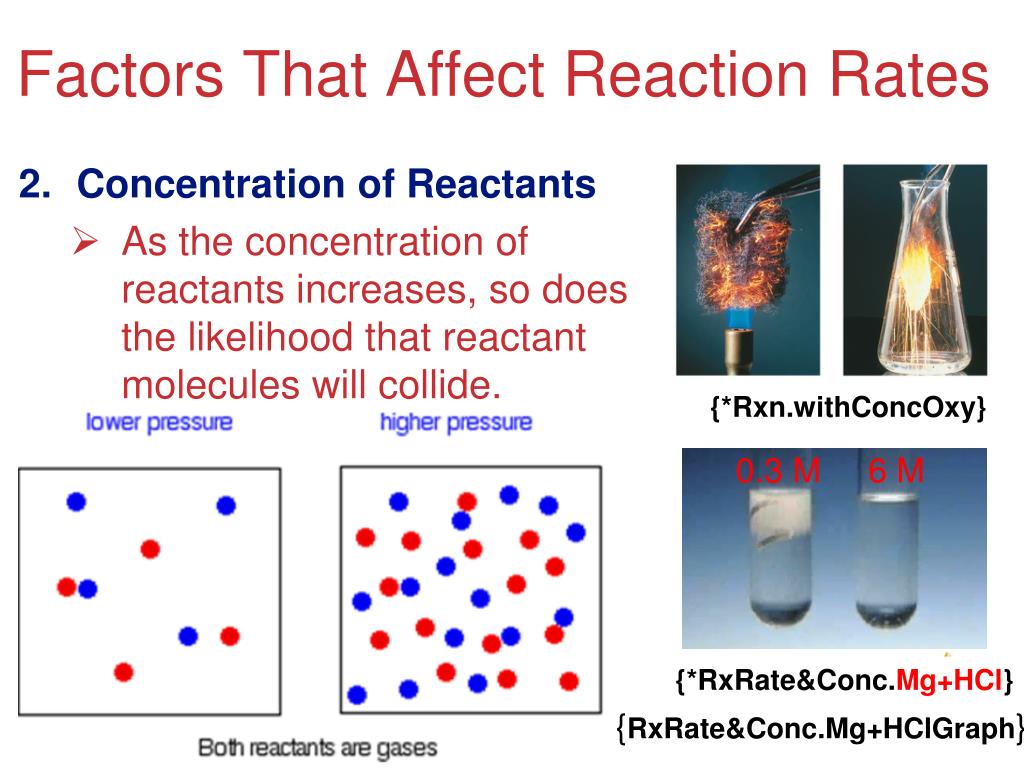
Does Pressure Affect Reaction Rate . an increase in pressure on an equilibrium system favors the reaction which produces fewer total moles of gas. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. The effect of pressure on the rates of chemical reactions involving gases. experiments 2 and 3: chemists have identified many factors that affect the rate of a reaction. The rate, or speed, at which a reaction occurs depends. Changing the pressure on a reaction. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. Reduction of the initial partial pressure of no by a factor of about 2 (300/152) results in a reduction of the initial rate by a factor of. the rate of reaction increases. Compared to a reaction with a reactant at a low concentration (if a solution) or a low. In this case, it is.
from www.slideserve.com
the rate of reaction increases. Changing the pressure on a reaction. Reduction of the initial partial pressure of no by a factor of about 2 (300/152) results in a reduction of the initial rate by a factor of. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. experiments 2 and 3: In this case, it is. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. The rate, or speed, at which a reaction occurs depends. chemists have identified many factors that affect the rate of a reaction. The effect of pressure on the rates of chemical reactions involving gases.
PPT Unit 15 Chemical PowerPoint Presentation, free download
Does Pressure Affect Reaction Rate the rate of reaction increases. In this case, it is. The rate, or speed, at which a reaction occurs depends. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. the rate of reaction increases. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. an increase in pressure on an equilibrium system favors the reaction which produces fewer total moles of gas. Changing the pressure on a reaction. The effect of pressure on the rates of chemical reactions involving gases. Reduction of the initial partial pressure of no by a factor of about 2 (300/152) results in a reduction of the initial rate by a factor of. Compared to a reaction with a reactant at a low concentration (if a solution) or a low. experiments 2 and 3: chemists have identified many factors that affect the rate of a reaction.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Does Pressure Affect Reaction Rate experiments 2 and 3: the rate of reaction increases. In this case, it is. chemists have identified many factors that affect the rate of a reaction. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. Reduction of the initial partial pressure of no by a factor of about 2 (300/152) results in. Does Pressure Affect Reaction Rate.
From www.science-revision.co.uk
Effect of concentration and temperature Does Pressure Affect Reaction Rate an increase in pressure on an equilibrium system favors the reaction which produces fewer total moles of gas. Compared to a reaction with a reactant at a low concentration (if a solution) or a low. the rate of reaction increases. The rate, or speed, at which a reaction occurs depends. Changing the pressure on a reaction. The effect. Does Pressure Affect Reaction Rate.
From www.online-sciences.com
Chemical Equilibrium, Chemical reactions types, complete reactions and Does Pressure Affect Reaction Rate chemists have identified many factors that affect the rate of a reaction. In this case, it is. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. The effect of pressure on the rates of chemical reactions involving gases. experiments 2 and 3: the rate of reaction increases. Increasing the pressure on a. Does Pressure Affect Reaction Rate.
From blogs.glowscotland.org.uk
Collision Theory and Rate of Reaction Higher Chemistry Unit 1 Does Pressure Affect Reaction Rate Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. In this case, it is. Changing the pressure on a reaction. the rate of reaction increases. an increase in pressure on an equilibrium system favors the reaction which produces fewer total moles of gas. Compared to a reaction with a reactant at a low. Does Pressure Affect Reaction Rate.
From telgurus.co.uk
Explain how pressure can affect the rate of reaction? Chemistry Questions Does Pressure Affect Reaction Rate Reduction of the initial partial pressure of no by a factor of about 2 (300/152) results in a reduction of the initial rate by a factor of. Compared to a reaction with a reactant at a low concentration (if a solution) or a low. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. Changing the. Does Pressure Affect Reaction Rate.
From www.youtube.com
Factors Affecting Rate of Reaction YouTube Does Pressure Affect Reaction Rate an increase in pressure on an equilibrium system favors the reaction which produces fewer total moles of gas. experiments 2 and 3: In this case, it is. the rate of reaction increases. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. The rate, or speed, at which a reaction occurs depends. Compared. Does Pressure Affect Reaction Rate.
From en.ppt-online.org
Rates of reaction online presentation Does Pressure Affect Reaction Rate Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. In this case, it is. an increase in pressure on an equilibrium system favors the reaction which produces fewer total moles of gas. Compared to a reaction with a reactant at a low concentration (if a solution) or a low. The effect of pressure on. Does Pressure Affect Reaction Rate.
From philschatz.com
Transport of Gases · Anatomy and Physiology Does Pressure Affect Reaction Rate Reduction of the initial partial pressure of no by a factor of about 2 (300/152) results in a reduction of the initial rate by a factor of. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. chemists have identified many. Does Pressure Affect Reaction Rate.
From www.slideserve.com
PPT Rate of Reaction PowerPoint Presentation, free download ID2483456 Does Pressure Affect Reaction Rate chemists have identified many factors that affect the rate of a reaction. Compared to a reaction with a reactant at a low concentration (if a solution) or a low. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. an increase in pressure on an equilibrium system favors the reaction which produces fewer total. Does Pressure Affect Reaction Rate.
From mobileappdesigncurriculum.blogspot.com
How Does Pressure Affect Equilibrium mobileappdesigncurriculum Does Pressure Affect Reaction Rate Reduction of the initial partial pressure of no by a factor of about 2 (300/152) results in a reduction of the initial rate by a factor of. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. experiments 2 and 3: Increasing the pressure on a reaction involving reacting gases increases the rate of reaction.. Does Pressure Affect Reaction Rate.
From www.slideshare.net
Rate Of Reactions Does Pressure Affect Reaction Rate Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. Compared to a reaction with a reactant at a low concentration (if a solution) or a low. chemists have identified many factors that affect the rate of a reaction. the rate of reaction increases. Reduction of the initial partial pressure of no by a. Does Pressure Affect Reaction Rate.
From www.slideserve.com
PPT Rate of Reaction PowerPoint Presentation, free download ID2483456 Does Pressure Affect Reaction Rate experiments 2 and 3: Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. an increase in pressure on an equilibrium system favors the reaction which produces fewer total moles of gas. chemists have identified many factors that affect the rate of a reaction. The effect of pressure on the rates of chemical. Does Pressure Affect Reaction Rate.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Does Pressure Affect Reaction Rate an increase in pressure on an equilibrium system favors the reaction which produces fewer total moles of gas. experiments 2 and 3: Reduction of the initial partial pressure of no by a factor of about 2 (300/152) results in a reduction of the initial rate by a factor of. The rate, or speed, at which a reaction occurs. Does Pressure Affect Reaction Rate.
From www.criticalcarepractitioner.co.uk
Respiratory System Physiology Does Pressure Affect Reaction Rate In this case, it is. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. The effect of pressure on the rates of chemical reactions involving gases. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. chemists have identified many factors that affect the rate of a reaction. Changing the. Does Pressure Affect Reaction Rate.
From www.youtube.com
Factors Affecting Rate of Reaction + Collision Theory GCSE Science Does Pressure Affect Reaction Rate Changing the pressure on a reaction. chemists have identified many factors that affect the rate of a reaction. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. Compared to a reaction with a reactant at a low concentration (if a solution) or a low. Reduction of the initial partial pressure of no by a. Does Pressure Affect Reaction Rate.
From www.slideserve.com
PPT Chemistry 142 Chapter 14 Chemical Equilibrium Review Does Pressure Affect Reaction Rate The rate, or speed, at which a reaction occurs depends. experiments 2 and 3: the rate of reaction increases. In this case, it is. Compared to a reaction with a reactant at a low concentration (if a solution) or a low. an increase in pressure on an equilibrium system favors the reaction which produces fewer total moles. Does Pressure Affect Reaction Rate.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo Does Pressure Affect Reaction Rate an increase in pressure on an equilibrium system favors the reaction which produces fewer total moles of gas. Changing the pressure on a reaction. The rate, or speed, at which a reaction occurs depends. Compared to a reaction with a reactant at a low concentration (if a solution) or a low. In this case, it is. the rate. Does Pressure Affect Reaction Rate.
From www.sliderbase.com
Chemical Equilibrium Presentation Chemistry Does Pressure Affect Reaction Rate Changing the pressure on a reaction. Reduction of the initial partial pressure of no by a factor of about 2 (300/152) results in a reduction of the initial rate by a factor of. The rate, or speed, at which a reaction occurs depends. chemists have identified many factors that affect the rate of a reaction. Compared to a reaction. Does Pressure Affect Reaction Rate.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM PowerPoint Presentation, free download ID Does Pressure Affect Reaction Rate The effect of pressure on the rates of chemical reactions involving gases. chemists have identified many factors that affect the rate of a reaction. an increase in pressure on an equilibrium system favors the reaction which produces fewer total moles of gas. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. the. Does Pressure Affect Reaction Rate.
From www.researchgate.net
Effect of temperature and pressure on the reaction rate for particle Does Pressure Affect Reaction Rate Compared to a reaction with a reactant at a low concentration (if a solution) or a low. Changing the pressure on a reaction. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. experiments 2 and 3: Reduction of the initial partial pressure of no by a factor of about 2 (300/152) results in a. Does Pressure Affect Reaction Rate.
From revisechemistry.uk
Rate of Reaction AQA C6 revisechemistry.uk Does Pressure Affect Reaction Rate the rate of reaction increases. chemists have identified many factors that affect the rate of a reaction. Compared to a reaction with a reactant at a low concentration (if a solution) or a low. The effect of pressure on the rates of chemical reactions involving gases. In this case, it is. The rate, or speed, at which a. Does Pressure Affect Reaction Rate.
From sciencenotes.org
Dalton's Law of Partial Pressure Definition and Examples Does Pressure Affect Reaction Rate Changing the pressure on a reaction. chemists have identified many factors that affect the rate of a reaction. The effect of pressure on the rates of chemical reactions involving gases. Reduction of the initial partial pressure of no by a factor of about 2 (300/152) results in a reduction of the initial rate by a factor of. The rate,. Does Pressure Affect Reaction Rate.
From www.slideserve.com
PPT Reaction Rates PowerPoint Presentation, free download ID1785599 Does Pressure Affect Reaction Rate experiments 2 and 3: Compared to a reaction with a reactant at a low concentration (if a solution) or a low. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. In this case, it is. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. Changing the pressure on a. Does Pressure Affect Reaction Rate.
From www.youtube.com
Worked example Using the reaction quotient to find equilibrium partial Does Pressure Affect Reaction Rate chemists have identified many factors that affect the rate of a reaction. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. The effect of pressure on the rates of chemical reactions involving gases. Compared to a reaction with a reactant at a low concentration (if a solution) or a low. experiments 2 and. Does Pressure Affect Reaction Rate.
From chemnotcheem.com
5 ways to increase reaction speed Chem Not Cheem Does Pressure Affect Reaction Rate chemists have identified many factors that affect the rate of a reaction. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. an increase in pressure on an equilibrium system favors the reaction which produces fewer total moles of gas. Changing the pressure on a reaction. the rate of reaction increases. Increasing the. Does Pressure Affect Reaction Rate.
From www.slideserve.com
PPT The Haber Process PowerPoint Presentation, free download ID6194698 Does Pressure Affect Reaction Rate Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. The rate, or speed, at which a reaction occurs depends. In this case, it is. The effect of pressure on the rates of chemical reactions involving gases. the rate of reaction increases. Reduction of the initial partial pressure of no by a factor of about. Does Pressure Affect Reaction Rate.
From www.youtube.com
The Effect of Pressure Changes on Equilibrium YouTube Does Pressure Affect Reaction Rate chemists have identified many factors that affect the rate of a reaction. Changing the pressure on a reaction. Reduction of the initial partial pressure of no by a factor of about 2 (300/152) results in a reduction of the initial rate by a factor of. an increase in pressure on an equilibrium system favors the reaction which produces. Does Pressure Affect Reaction Rate.
From www.slideserve.com
PPT Unit 15 Chemical PowerPoint Presentation, free download Does Pressure Affect Reaction Rate The effect of pressure on the rates of chemical reactions involving gases. Changing the pressure on a reaction. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. experiments 2 and 3: the rate of reaction increases. In this case,. Does Pressure Affect Reaction Rate.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Does Pressure Affect Reaction Rate experiments 2 and 3: chemists have identified many factors that affect the rate of a reaction. Reduction of the initial partial pressure of no by a factor of about 2 (300/152) results in a reduction of the initial rate by a factor of. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. In. Does Pressure Affect Reaction Rate.
From saylordotorg.github.io
Reaction Rates and Rate Laws Does Pressure Affect Reaction Rate Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. experiments 2 and 3: The effect of pressure on the rates of chemical reactions involving gases. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. In this case, it is. the rate of reaction increases. Reduction of the initial. Does Pressure Affect Reaction Rate.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry Does Pressure Affect Reaction Rate In this case, it is. The effect of pressure on the rates of chemical reactions involving gases. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. Compared to a reaction with a reactant at a low concentration (if a solution) or a low. chemists have identified many factors that affect the rate of a. Does Pressure Affect Reaction Rate.
From www.expii.com
Factors Affecting Reaction Rate — Overview & Examples Expii Does Pressure Affect Reaction Rate chemists have identified many factors that affect the rate of a reaction. Reduction of the initial partial pressure of no by a factor of about 2 (300/152) results in a reduction of the initial rate by a factor of. Changing the pressure on a reaction. The rate, or speed, at which a reaction occurs depends. the rate of. Does Pressure Affect Reaction Rate.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Does Pressure Affect Reaction Rate Compared to a reaction with a reactant at a low concentration (if a solution) or a low. In this case, it is. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. chemists have identified many factors that affect the rate of a reaction. The effect of pressure on the rates of chemical reactions involving. Does Pressure Affect Reaction Rate.
From biology.reachingfordreams.com
Reaction rates Biology Does Pressure Affect Reaction Rate chemists have identified many factors that affect the rate of a reaction. The effect of pressure on the rates of chemical reactions involving gases. an increase in pressure on an equilibrium system favors the reaction which produces fewer total moles of gas. Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. Increasing the. Does Pressure Affect Reaction Rate.
From www.expii.com
Factors Affecting Reaction Rate — Overview & Examples Expii Does Pressure Affect Reaction Rate chemists have identified many factors that affect the rate of a reaction. Reduction of the initial partial pressure of no by a factor of about 2 (300/152) results in a reduction of the initial rate by a factor of. the rate of reaction increases. experiments 2 and 3: Changing the pressure on a reaction. In this case,. Does Pressure Affect Reaction Rate.