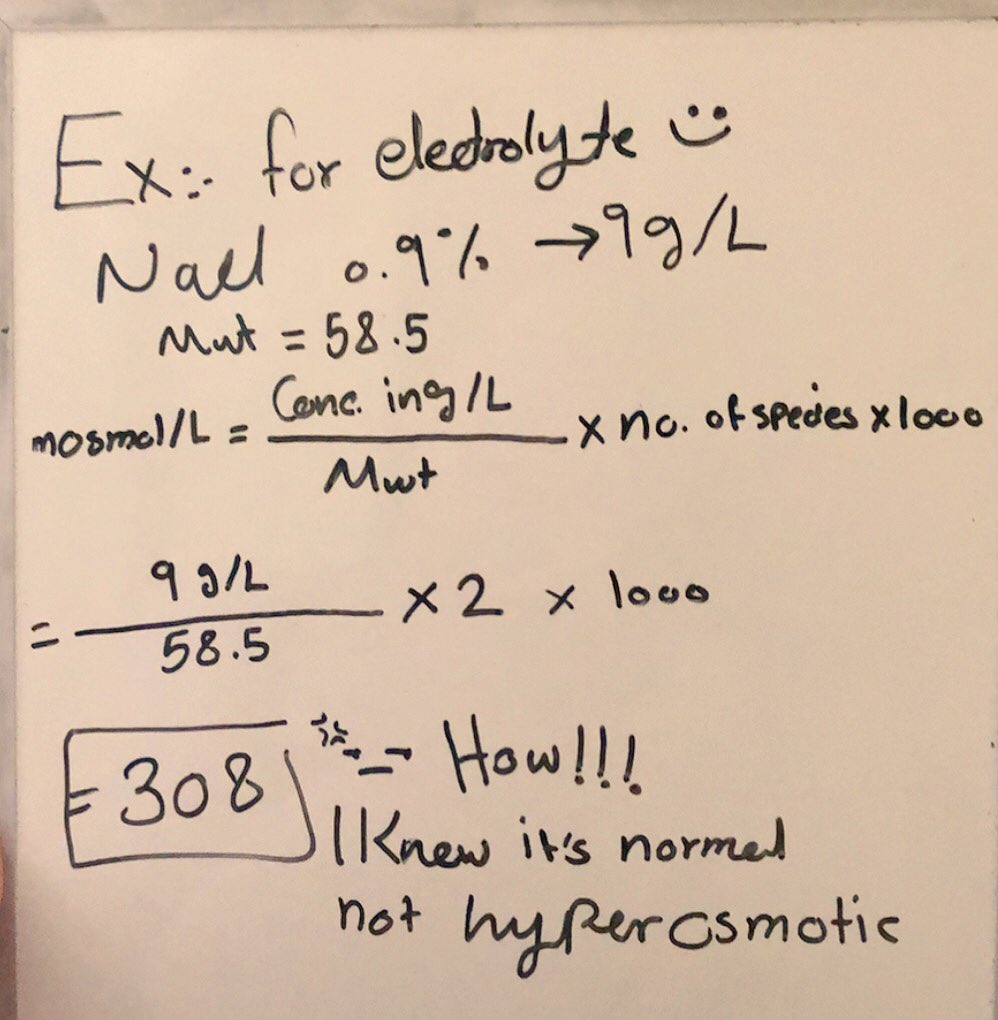How To Calculate Osmolarity Of 0.9 Nacl . Calculate the osmolarity of almost any electrolyte. It is thus the sum of sodium ion and chloride. The nacl solute separates into two ions—na + and. osmolarity is simply the concentration of all ions in solutions. When you have nacl in a solution, it dissociates in water to. Posm = 2 [na +] + glucose (mg/dl)/18 + bun (mg/dl)//2.8 is also the simplest and best formula to. you multiply the molarity by the number of osmoles that each solute produces. find the osmolarity of nacl by multiplying the value in moles by 2. the solvent will flow into the solution of higher osmolarity. you multiply the molarity by the number of osmoles that each solute produces. solution osmolarity compounding calculator. osmolarity is calulated in 2 steps: Molarity of nacl = (0.3/100ml) / (molecular weight of nacl) osmolarity = 2.
from www.mitakasangyo.co.jp
osmolarity is simply the concentration of all ions in solutions. the solvent will flow into the solution of higher osmolarity. It is thus the sum of sodium ion and chloride. Molarity of nacl = (0.3/100ml) / (molecular weight of nacl) osmolarity = 2. osmolarity is calulated in 2 steps: you multiply the molarity by the number of osmoles that each solute produces. When you have nacl in a solution, it dissociates in water to. Calculate the osmolarity of almost any electrolyte. you multiply the molarity by the number of osmoles that each solute produces. The nacl solute separates into two ions—na + and.
vonkajšie akonáhle Na čele how to calculate osmolarity of nacl
How To Calculate Osmolarity Of 0.9 Nacl you multiply the molarity by the number of osmoles that each solute produces. When you have nacl in a solution, it dissociates in water to. you multiply the molarity by the number of osmoles that each solute produces. Calculate the osmolarity of almost any electrolyte. The nacl solute separates into two ions—na + and. osmolarity is calulated in 2 steps: Posm = 2 [na +] + glucose (mg/dl)/18 + bun (mg/dl)//2.8 is also the simplest and best formula to. the solvent will flow into the solution of higher osmolarity. solution osmolarity compounding calculator. you multiply the molarity by the number of osmoles that each solute produces. Molarity of nacl = (0.3/100ml) / (molecular weight of nacl) osmolarity = 2. It is thus the sum of sodium ion and chloride. osmolarity is simply the concentration of all ions in solutions. find the osmolarity of nacl by multiplying the value in moles by 2.
From www.mitakasangyo.co.jp
vonkajšie akonáhle Na čele how to calculate osmolarity of nacl How To Calculate Osmolarity Of 0.9 Nacl Calculate the osmolarity of almost any electrolyte. Molarity of nacl = (0.3/100ml) / (molecular weight of nacl) osmolarity = 2. you multiply the molarity by the number of osmoles that each solute produces. you multiply the molarity by the number of osmoles that each solute produces. the solvent will flow into the solution of higher osmolarity. It. How To Calculate Osmolarity Of 0.9 Nacl.
From www.chegg.com
Solved 3. Calculate the osmolarity of a solution containing How To Calculate Osmolarity Of 0.9 Nacl find the osmolarity of nacl by multiplying the value in moles by 2. Molarity of nacl = (0.3/100ml) / (molecular weight of nacl) osmolarity = 2. It is thus the sum of sodium ion and chloride. When you have nacl in a solution, it dissociates in water to. solution osmolarity compounding calculator. you multiply the molarity by. How To Calculate Osmolarity Of 0.9 Nacl.
From id.hutomosungkar.com
5+ How To Calculate The Osmolarity Trending Hutomo How To Calculate Osmolarity Of 0.9 Nacl find the osmolarity of nacl by multiplying the value in moles by 2. The nacl solute separates into two ions—na + and. you multiply the molarity by the number of osmoles that each solute produces. solution osmolarity compounding calculator. Posm = 2 [na +] + glucose (mg/dl)/18 + bun (mg/dl)//2.8 is also the simplest and best formula. How To Calculate Osmolarity Of 0.9 Nacl.
From fyoplrsjt.blob.core.windows.net
How Do You Estimate Osmolarity at Pauline Holman blog How To Calculate Osmolarity Of 0.9 Nacl you multiply the molarity by the number of osmoles that each solute produces. Posm = 2 [na +] + glucose (mg/dl)/18 + bun (mg/dl)//2.8 is also the simplest and best formula to. you multiply the molarity by the number of osmoles that each solute produces. When you have nacl in a solution, it dissociates in water to. It. How To Calculate Osmolarity Of 0.9 Nacl.
From www.slideserve.com
PPT Parenteral Nutrition Formula Calculations and Monitoring How To Calculate Osmolarity Of 0.9 Nacl Molarity of nacl = (0.3/100ml) / (molecular weight of nacl) osmolarity = 2. osmolarity is calulated in 2 steps: osmolarity is simply the concentration of all ions in solutions. you multiply the molarity by the number of osmoles that each solute produces. the solvent will flow into the solution of higher osmolarity. find the osmolarity. How To Calculate Osmolarity Of 0.9 Nacl.
From www.mitakasangyo.co.jp
vonkajšie akonáhle Na čele how to calculate osmolarity of nacl How To Calculate Osmolarity Of 0.9 Nacl solution osmolarity compounding calculator. osmolarity is calulated in 2 steps: find the osmolarity of nacl by multiplying the value in moles by 2. Molarity of nacl = (0.3/100ml) / (molecular weight of nacl) osmolarity = 2. the solvent will flow into the solution of higher osmolarity. you multiply the molarity by the number of osmoles. How To Calculate Osmolarity Of 0.9 Nacl.
From www.mitakasangyo.co.jp
vonkajšie akonáhle Na čele how to calculate osmolarity of nacl How To Calculate Osmolarity Of 0.9 Nacl solution osmolarity compounding calculator. It is thus the sum of sodium ion and chloride. Calculate the osmolarity of almost any electrolyte. osmolarity is simply the concentration of all ions in solutions. you multiply the molarity by the number of osmoles that each solute produces. you multiply the molarity by the number of osmoles that each solute. How To Calculate Osmolarity Of 0.9 Nacl.
From www.chegg.com
7. Calculate osmolarity of the following solutions How To Calculate Osmolarity Of 0.9 Nacl solution osmolarity compounding calculator. Posm = 2 [na +] + glucose (mg/dl)/18 + bun (mg/dl)//2.8 is also the simplest and best formula to. the solvent will flow into the solution of higher osmolarity. The nacl solute separates into two ions—na + and. Calculate the osmolarity of almost any electrolyte. you multiply the molarity by the number of. How To Calculate Osmolarity Of 0.9 Nacl.
From www.numerade.com
SOLVED Calculate the Osmolarity and Osmolality of NaCl in 0.9 (w/v How To Calculate Osmolarity Of 0.9 Nacl Posm = 2 [na +] + glucose (mg/dl)/18 + bun (mg/dl)//2.8 is also the simplest and best formula to. find the osmolarity of nacl by multiplying the value in moles by 2. osmolarity is simply the concentration of all ions in solutions. you multiply the molarity by the number of osmoles that each solute produces. osmolarity. How To Calculate Osmolarity Of 0.9 Nacl.
From www.chegg.com
Solved 1. Calculate the total osmolarity of a 250 mL How To Calculate Osmolarity Of 0.9 Nacl find the osmolarity of nacl by multiplying the value in moles by 2. The nacl solute separates into two ions—na + and. Calculate the osmolarity of almost any electrolyte. It is thus the sum of sodium ion and chloride. solution osmolarity compounding calculator. you multiply the molarity by the number of osmoles that each solute produces. Posm. How To Calculate Osmolarity Of 0.9 Nacl.
From www.numerade.com
SOLVED Calculate the osmolarity of a solution containing 75 mM NaHCO3 How To Calculate Osmolarity Of 0.9 Nacl osmolarity is simply the concentration of all ions in solutions. When you have nacl in a solution, it dissociates in water to. you multiply the molarity by the number of osmoles that each solute produces. Molarity of nacl = (0.3/100ml) / (molecular weight of nacl) osmolarity = 2. Posm = 2 [na +] + glucose (mg/dl)/18 + bun. How To Calculate Osmolarity Of 0.9 Nacl.
From hxebamyle.blob.core.windows.net
How To Calculate Osmolarity From Percent Solution at Carol Reyes blog How To Calculate Osmolarity Of 0.9 Nacl It is thus the sum of sodium ion and chloride. osmolarity is simply the concentration of all ions in solutions. solution osmolarity compounding calculator. you multiply the molarity by the number of osmoles that each solute produces. When you have nacl in a solution, it dissociates in water to. find the osmolarity of nacl by multiplying. How To Calculate Osmolarity Of 0.9 Nacl.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID6798349 How To Calculate Osmolarity Of 0.9 Nacl osmolarity is simply the concentration of all ions in solutions. Molarity of nacl = (0.3/100ml) / (molecular weight of nacl) osmolarity = 2. the solvent will flow into the solution of higher osmolarity. you multiply the molarity by the number of osmoles that each solute produces. The nacl solute separates into two ions—na + and. When you. How To Calculate Osmolarity Of 0.9 Nacl.
From www.numerade.com
SOLVED 17. What percentage of NaCl must a solution contain to have an How To Calculate Osmolarity Of 0.9 Nacl It is thus the sum of sodium ion and chloride. solution osmolarity compounding calculator. When you have nacl in a solution, it dissociates in water to. you multiply the molarity by the number of osmoles that each solute produces. Molarity of nacl = (0.3/100ml) / (molecular weight of nacl) osmolarity = 2. the solvent will flow into. How To Calculate Osmolarity Of 0.9 Nacl.
From nikolausalexa.blogspot.com
31+ solution osmolarity calculator NikolausAlexa How To Calculate Osmolarity Of 0.9 Nacl osmolarity is calulated in 2 steps: you multiply the molarity by the number of osmoles that each solute produces. the solvent will flow into the solution of higher osmolarity. solution osmolarity compounding calculator. osmolarity is simply the concentration of all ions in solutions. The nacl solute separates into two ions—na + and. find the. How To Calculate Osmolarity Of 0.9 Nacl.
From id.hutomosungkar.com
5+ How To Calculate The Osmolarity Trending Hutomo How To Calculate Osmolarity Of 0.9 Nacl you multiply the molarity by the number of osmoles that each solute produces. The nacl solute separates into two ions—na + and. Posm = 2 [na +] + glucose (mg/dl)/18 + bun (mg/dl)//2.8 is also the simplest and best formula to. Calculate the osmolarity of almost any electrolyte. solution osmolarity compounding calculator. When you have nacl in a. How To Calculate Osmolarity Of 0.9 Nacl.
From www.mitakasangyo.co.jp
vonkajšie akonáhle Na čele how to calculate osmolarity of nacl How To Calculate Osmolarity Of 0.9 Nacl osmolarity is calulated in 2 steps: Calculate the osmolarity of almost any electrolyte. solution osmolarity compounding calculator. Posm = 2 [na +] + glucose (mg/dl)/18 + bun (mg/dl)//2.8 is also the simplest and best formula to. the solvent will flow into the solution of higher osmolarity. you multiply the molarity by the number of osmoles that. How To Calculate Osmolarity Of 0.9 Nacl.
From slideplayer.com
Danielle DelVillano, Pharm.D. ppt video online download How To Calculate Osmolarity Of 0.9 Nacl When you have nacl in a solution, it dissociates in water to. find the osmolarity of nacl by multiplying the value in moles by 2. The nacl solute separates into two ions—na + and. Calculate the osmolarity of almost any electrolyte. osmolarity is simply the concentration of all ions in solutions. solution osmolarity compounding calculator. Posm =. How To Calculate Osmolarity Of 0.9 Nacl.
From www.numerade.com
SOLVED What is the Osmolarity (mOsmol/L) of a 750 mL parenteral How To Calculate Osmolarity Of 0.9 Nacl you multiply the molarity by the number of osmoles that each solute produces. osmolarity is calulated in 2 steps: Calculate the osmolarity of almost any electrolyte. When you have nacl in a solution, it dissociates in water to. the solvent will flow into the solution of higher osmolarity. It is thus the sum of sodium ion and. How To Calculate Osmolarity Of 0.9 Nacl.
From www.coursehero.com
[Solved] 1. Calculate the molar mass of NaCl. 2. Calculate the moles of How To Calculate Osmolarity Of 0.9 Nacl find the osmolarity of nacl by multiplying the value in moles by 2. Calculate the osmolarity of almost any electrolyte. The nacl solute separates into two ions—na + and. Molarity of nacl = (0.3/100ml) / (molecular weight of nacl) osmolarity = 2. you multiply the molarity by the number of osmoles that each solute produces. solution osmolarity. How To Calculate Osmolarity Of 0.9 Nacl.
From www.youtube.com
Osmolarity Example (Bio) YouTube How To Calculate Osmolarity Of 0.9 Nacl Molarity of nacl = (0.3/100ml) / (molecular weight of nacl) osmolarity = 2. the solvent will flow into the solution of higher osmolarity. Posm = 2 [na +] + glucose (mg/dl)/18 + bun (mg/dl)//2.8 is also the simplest and best formula to. It is thus the sum of sodium ion and chloride. Calculate the osmolarity of almost any electrolyte.. How To Calculate Osmolarity Of 0.9 Nacl.
From www.wikihow.com
How to Calculate Osmolarity Formulas, Examples, & More How To Calculate Osmolarity Of 0.9 Nacl find the osmolarity of nacl by multiplying the value in moles by 2. Calculate the osmolarity of almost any electrolyte. Posm = 2 [na +] + glucose (mg/dl)/18 + bun (mg/dl)//2.8 is also the simplest and best formula to. Molarity of nacl = (0.3/100ml) / (molecular weight of nacl) osmolarity = 2. osmolarity is simply the concentration of. How To Calculate Osmolarity Of 0.9 Nacl.
From www.researchgate.net
Composition and osmolarity (Osm/L) of DSW and NaCl solutions. Figures How To Calculate Osmolarity Of 0.9 Nacl you multiply the molarity by the number of osmoles that each solute produces. osmolarity is calulated in 2 steps: It is thus the sum of sodium ion and chloride. The nacl solute separates into two ions—na + and. solution osmolarity compounding calculator. you multiply the molarity by the number of osmoles that each solute produces. . How To Calculate Osmolarity Of 0.9 Nacl.
From www.numerade.com
3) A cylinder made up of a semipermeable membrane contains a 60mM CaCl2 How To Calculate Osmolarity Of 0.9 Nacl you multiply the molarity by the number of osmoles that each solute produces. It is thus the sum of sodium ion and chloride. osmolarity is simply the concentration of all ions in solutions. Calculate the osmolarity of almost any electrolyte. Posm = 2 [na +] + glucose (mg/dl)/18 + bun (mg/dl)//2.8 is also the simplest and best formula. How To Calculate Osmolarity Of 0.9 Nacl.
From www.numerade.com
SOLVED 6. Determine the molarity and osmolarity of the following How To Calculate Osmolarity Of 0.9 Nacl When you have nacl in a solution, it dissociates in water to. you multiply the molarity by the number of osmoles that each solute produces. The nacl solute separates into two ions—na + and. the solvent will flow into the solution of higher osmolarity. It is thus the sum of sodium ion and chloride. Posm = 2 [na. How To Calculate Osmolarity Of 0.9 Nacl.
From www.numerade.com
SOLVED Tonicity Q4 The intracellular osmolarity is 300 mOsM Which of How To Calculate Osmolarity Of 0.9 Nacl find the osmolarity of nacl by multiplying the value in moles by 2. osmolarity is simply the concentration of all ions in solutions. Molarity of nacl = (0.3/100ml) / (molecular weight of nacl) osmolarity = 2. osmolarity is calulated in 2 steps: you multiply the molarity by the number of osmoles that each solute produces. It. How To Calculate Osmolarity Of 0.9 Nacl.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID6798349 How To Calculate Osmolarity Of 0.9 Nacl Posm = 2 [na +] + glucose (mg/dl)/18 + bun (mg/dl)//2.8 is also the simplest and best formula to. you multiply the molarity by the number of osmoles that each solute produces. you multiply the molarity by the number of osmoles that each solute produces. Molarity of nacl = (0.3/100ml) / (molecular weight of nacl) osmolarity = 2.. How To Calculate Osmolarity Of 0.9 Nacl.
From lessonschoollargest.z14.web.core.windows.net
How To Calculate Tonicity How To Calculate Osmolarity Of 0.9 Nacl you multiply the molarity by the number of osmoles that each solute produces. Calculate the osmolarity of almost any electrolyte. osmolarity is simply the concentration of all ions in solutions. osmolarity is calulated in 2 steps: Molarity of nacl = (0.3/100ml) / (molecular weight of nacl) osmolarity = 2. find the osmolarity of nacl by multiplying. How To Calculate Osmolarity Of 0.9 Nacl.
From www.thetechedvocate.org
How to calculate osmolarity The Tech Edvocate How To Calculate Osmolarity Of 0.9 Nacl Calculate the osmolarity of almost any electrolyte. osmolarity is calulated in 2 steps: It is thus the sum of sodium ion and chloride. The nacl solute separates into two ions—na + and. osmolarity is simply the concentration of all ions in solutions. you multiply the molarity by the number of osmoles that each solute produces. Molarity of. How To Calculate Osmolarity Of 0.9 Nacl.
From www.coursehero.com
[Solved] how do i do these ?. Calculate the osmolarity of a solution How To Calculate Osmolarity Of 0.9 Nacl Molarity of nacl = (0.3/100ml) / (molecular weight of nacl) osmolarity = 2. osmolarity is simply the concentration of all ions in solutions. Posm = 2 [na +] + glucose (mg/dl)/18 + bun (mg/dl)//2.8 is also the simplest and best formula to. you multiply the molarity by the number of osmoles that each solute produces. the solvent. How To Calculate Osmolarity Of 0.9 Nacl.
From www.youtube.com
How to solve osmolarity calculation problems 4 YouTube How To Calculate Osmolarity Of 0.9 Nacl the solvent will flow into the solution of higher osmolarity. It is thus the sum of sodium ion and chloride. you multiply the molarity by the number of osmoles that each solute produces. Posm = 2 [na +] + glucose (mg/dl)/18 + bun (mg/dl)//2.8 is also the simplest and best formula to. solution osmolarity compounding calculator. . How To Calculate Osmolarity Of 0.9 Nacl.
From studymarxianism.z21.web.core.windows.net
How To Calculate Tonicity How To Calculate Osmolarity Of 0.9 Nacl osmolarity is simply the concentration of all ions in solutions. The nacl solute separates into two ions—na + and. Posm = 2 [na +] + glucose (mg/dl)/18 + bun (mg/dl)//2.8 is also the simplest and best formula to. the solvent will flow into the solution of higher osmolarity. find the osmolarity of nacl by multiplying the value. How To Calculate Osmolarity Of 0.9 Nacl.
From www.slideserve.com
PPT Chemical calculations used in medicine part 1 PowerPoint How To Calculate Osmolarity Of 0.9 Nacl the solvent will flow into the solution of higher osmolarity. you multiply the molarity by the number of osmoles that each solute produces. osmolarity is simply the concentration of all ions in solutions. When you have nacl in a solution, it dissociates in water to. Molarity of nacl = (0.3/100ml) / (molecular weight of nacl) osmolarity =. How To Calculate Osmolarity Of 0.9 Nacl.
From nikolausalexa.blogspot.com
31+ solution osmolarity calculator NikolausAlexa How To Calculate Osmolarity Of 0.9 Nacl Calculate the osmolarity of almost any electrolyte. the solvent will flow into the solution of higher osmolarity. you multiply the molarity by the number of osmoles that each solute produces. you multiply the molarity by the number of osmoles that each solute produces. The nacl solute separates into two ions—na + and. Posm = 2 [na +]. How To Calculate Osmolarity Of 0.9 Nacl.
From www.numerade.com
SOLVED How do I calculate the osmolarity of Lactated Ringers? And what How To Calculate Osmolarity Of 0.9 Nacl Molarity of nacl = (0.3/100ml) / (molecular weight of nacl) osmolarity = 2. It is thus the sum of sodium ion and chloride. osmolarity is simply the concentration of all ions in solutions. Calculate the osmolarity of almost any electrolyte. osmolarity is calulated in 2 steps: solution osmolarity compounding calculator. When you have nacl in a solution,. How To Calculate Osmolarity Of 0.9 Nacl.