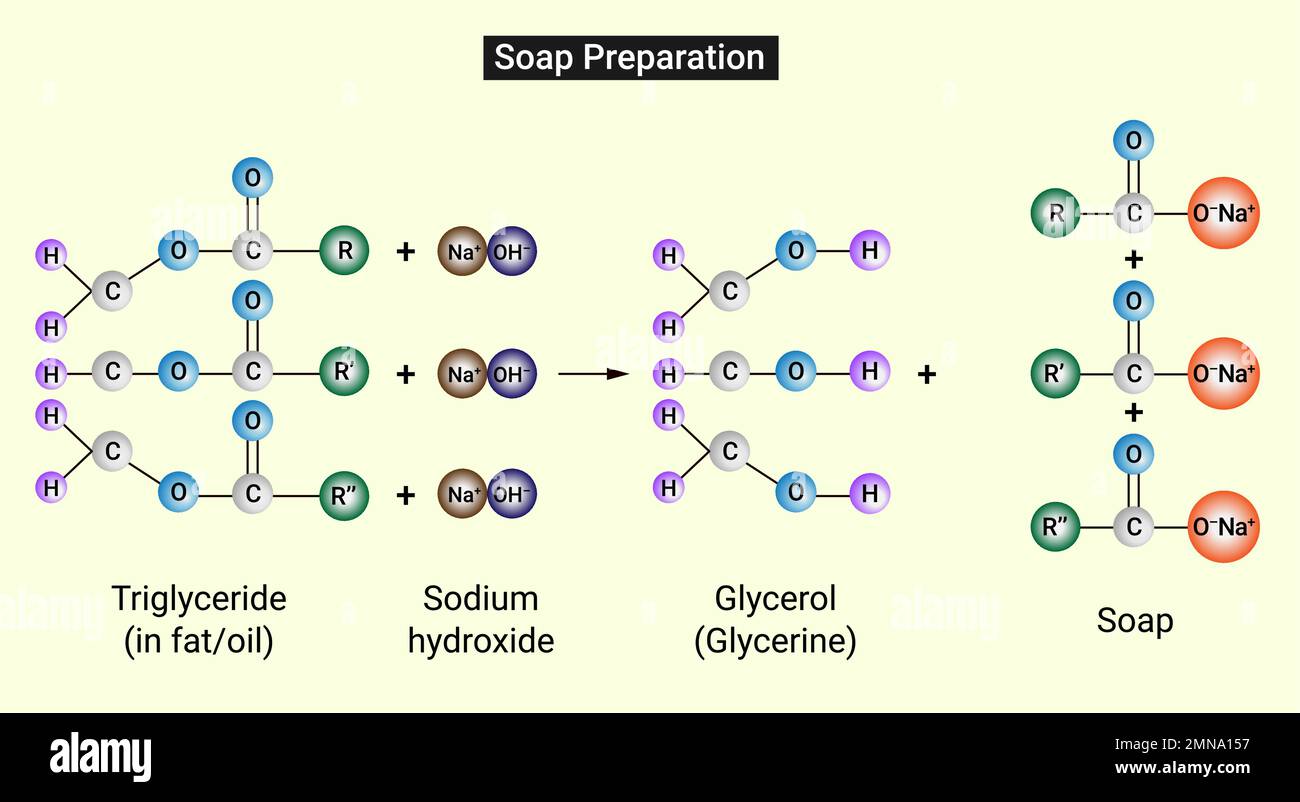
Method Of Making Soap With Chemical Equation . The chemical formula of the soap is ch3(ch2)14coona+. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Devise an experimental procedure to test the chemical properties of soap. Circle the portion of the molecule that. Explain why soap has the ability to clean greasy surfaces or stains. Try this class practical to make a soap or detergent using castor oil and either sodium hydroxide or sulfuric acid. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye or potash (potassium hydroxide). Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. One the above structure, circle. Includes kit list and safety instructions. The chemical structures of fats and oils generally look like this: You will precipitate the soap by adding it to a.
from ar.inspiredpencil.com
Try this class practical to make a soap or detergent using castor oil and either sodium hydroxide or sulfuric acid. Includes kit list and safety instructions. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye or potash (potassium hydroxide). You will precipitate the soap by adding it to a. The chemical structures of fats and oils generally look like this: In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. Devise an experimental procedure to test the chemical properties of soap. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. The chemical formula of the soap is ch3(ch2)14coona+. Circle the portion of the molecule that.
Preparation Of Soap Chemistry
Method Of Making Soap With Chemical Equation The chemical structures of fats and oils generally look like this: You will precipitate the soap by adding it to a. Devise an experimental procedure to test the chemical properties of soap. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye or potash (potassium hydroxide). Circle the portion of the molecule that. Explain why soap has the ability to clean greasy surfaces or stains. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). One the above structure, circle. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Try this class practical to make a soap or detergent using castor oil and either sodium hydroxide or sulfuric acid. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. Includes kit list and safety instructions. The chemical structures of fats and oils generally look like this: The chemical formula of the soap is ch3(ch2)14coona+.
From mungfali.com
Illustrated Glossary Of Organic Chemistry Soap 0CB Method Of Making Soap With Chemical Equation Try this class practical to make a soap or detergent using castor oil and either sodium hydroxide or sulfuric acid. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye or potash (potassium hydroxide). Devise an experimental procedure to test the chemical properties of soap. Explain why soap. Method Of Making Soap With Chemical Equation.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Method Of Making Soap With Chemical Equation Try this class practical to make a soap or detergent using castor oil and either sodium hydroxide or sulfuric acid. Circle the portion of the molecule that. One the above structure, circle. You will precipitate the soap by adding it to a. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction. Method Of Making Soap With Chemical Equation.
From ar.inspiredpencil.com
Preparation Of Soap Chemistry Method Of Making Soap With Chemical Equation The chemical formula of the soap is ch3(ch2)14coona+. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye or potash (potassium hydroxide). Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). You will precipitate the soap. Method Of Making Soap With Chemical Equation.
From www.thoughtco.com
How Saponification Makes Soap Method Of Making Soap With Chemical Equation Includes kit list and safety instructions. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Circle the portion of the molecule that. One the above structure, circle. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye. Method Of Making Soap With Chemical Equation.
From www.slideshare.net
Chemistry of soaps Method Of Making Soap With Chemical Equation Try this class practical to make a soap or detergent using castor oil and either sodium hydroxide or sulfuric acid. Devise an experimental procedure to test the chemical properties of soap. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. One the above structure, circle. Natural soaps are sodium. Method Of Making Soap With Chemical Equation.
From www.slideserve.com
PPT How is Soap Made? PowerPoint Presentation ID2530430 Method Of Making Soap With Chemical Equation Includes kit list and safety instructions. You will precipitate the soap by adding it to a. Try this class practical to make a soap or detergent using castor oil and either sodium hydroxide or sulfuric acid. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). One the above. Method Of Making Soap With Chemical Equation.
From www.researchgate.net
Flow diagram of soap production by bath process. Download Scientific Method Of Making Soap With Chemical Equation The chemical structures of fats and oils generally look like this: Explain why soap has the ability to clean greasy surfaces or stains. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye or potash (potassium hydroxide). One the above structure, circle. Includes kit list and safety instructions.. Method Of Making Soap With Chemical Equation.
From spmchemistry.blog.onlinetuition.com.my
The Making of Detergent SPM Chemistry Method Of Making Soap With Chemical Equation The chemical formula of the soap is ch3(ch2)14coona+. Explain why soap has the ability to clean greasy surfaces or stains. Try this class practical to make a soap or detergent using castor oil and either sodium hydroxide or sulfuric acid. Devise an experimental procedure to test the chemical properties of soap. Includes kit list and safety instructions. In this experiment,. Method Of Making Soap With Chemical Equation.
From ar.inspiredpencil.com
Preparation Of Soap In Chemistry Project Method Of Making Soap With Chemical Equation In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. Includes kit list and safety instructions. Explain why soap has the ability to clean greasy surfaces or stains. The chemical structures of fats and oils generally look like this: One the above structure, circle. The chemical formula of the soap is. Method Of Making Soap With Chemical Equation.
From ar.inspiredpencil.com
Soap Molecule Structure Method Of Making Soap With Chemical Equation Circle the portion of the molecule that. The chemical formula of the soap is ch3(ch2)14coona+. Includes kit list and safety instructions. One the above structure, circle. You will precipitate the soap by adding it to a. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). Natural soaps are. Method Of Making Soap With Chemical Equation.
From www.shutterstock.com
Soap Chemistry Soap Chemistry Formula Structure Stock Vector (Royalty Method Of Making Soap With Chemical Equation In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. You will precipitate the soap by adding it to a. Circle the portion of the molecule that. Devise an experimental procedure to test the chemical properties of soap. The chemical formula of the soap is ch3(ch2)14coona+. One the above structure, circle.. Method Of Making Soap With Chemical Equation.
From www.numerade.com
SOLVED Write an overall balanced chemical equation for the formation Method Of Making Soap With Chemical Equation Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). Explain why soap has the ability to clean greasy surfaces or stains. One the above structure, circle. Try this class practical to make a soap or detergent using castor oil and either sodium hydroxide or sulfuric acid. Natural soaps. Method Of Making Soap With Chemical Equation.
From thesoapmoleculeco.com
The Soap Molecule Co. Method Of Making Soap With Chemical Equation The chemical formula of the soap is ch3(ch2)14coona+. The chemical structures of fats and oils generally look like this: Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). Explain why soap has the ability to clean greasy surfaces or stains. One the above structure, circle. You will precipitate. Method Of Making Soap With Chemical Equation.
From ar.inspiredpencil.com
Preparation Of Soap Chemistry Method Of Making Soap With Chemical Equation One the above structure, circle. Try this class practical to make a soap or detergent using castor oil and either sodium hydroxide or sulfuric acid. Explain why soap has the ability to clean greasy surfaces or stains. Devise an experimental procedure to test the chemical properties of soap. In this experiment, you will make soap from a fat or an. Method Of Making Soap With Chemical Equation.
From hxewhpfvt.blob.core.windows.net
Soap Making Equation at Edna Fields blog Method Of Making Soap With Chemical Equation In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye or potash (potassium hydroxide). The chemical formula of the soap is ch3(ch2)14coona+. One the above structure, circle. Try this. Method Of Making Soap With Chemical Equation.
From studylib.net
Soap and Saponification Method Of Making Soap With Chemical Equation One the above structure, circle. Devise an experimental procedure to test the chemical properties of soap. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye or potash. Method Of Making Soap With Chemical Equation.
From labmuffin.com
Make Your Own Soap! Part 1 The Chemistry Behind Soap Making Lab Method Of Making Soap With Chemical Equation In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with. Method Of Making Soap With Chemical Equation.
From www.scribd.com
The Chemical Reaction of Soap Making Chemistry Physical Sciences Method Of Making Soap With Chemical Equation The chemical formula of the soap is ch3(ch2)14coona+. Explain why soap has the ability to clean greasy surfaces or stains. Includes kit list and safety instructions. Devise an experimental procedure to test the chemical properties of soap. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye or. Method Of Making Soap With Chemical Equation.
From www.teachoo.com
[Class 10] Soaps and Detergents Structure, Cleansing Action and more Method Of Making Soap With Chemical Equation Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye or potash (potassium hydroxide). The chemical structures of fats and oils generally look like this: Includes kit list and safety instructions. Explain why soap has the ability to clean greasy surfaces or stains. You will precipitate the soap. Method Of Making Soap With Chemical Equation.
From www.shutterstock.com
Saponification Equation Reaction Soap Chemistry Equation Stock Method Of Making Soap With Chemical Equation Try this class practical to make a soap or detergent using castor oil and either sodium hydroxide or sulfuric acid. The chemical formula of the soap is ch3(ch2)14coona+. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. Explain why soap has the ability to clean greasy surfaces or stains. Circle. Method Of Making Soap With Chemical Equation.
From lessonlibwheelbases.z22.web.core.windows.net
Soap Equation Math Method Of Making Soap With Chemical Equation Circle the portion of the molecule that. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). Try this class practical to make a soap or detergent using castor oil and either sodium hydroxide or sulfuric acid. One of the organic chemical reactions known to ancient man was the. Method Of Making Soap With Chemical Equation.
From www.pinterest.com
Saponification explanation. Soap making, Chemistry, Soap Method Of Making Soap With Chemical Equation Explain why soap has the ability to clean greasy surfaces or stains. One the above structure, circle. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Includes kit list and safety instructions. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other. Method Of Making Soap With Chemical Equation.
From www.pinterest.com
An Introduction to Chemistry Soap, Soap making, Chemical reactions Method Of Making Soap With Chemical Equation Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide.. Method Of Making Soap With Chemical Equation.
From www.youtube.com
Hydrolysis & How It Is Used To Make Soaps Organic Chemistry Method Of Making Soap With Chemical Equation The chemical formula of the soap is ch3(ch2)14coona+. One the above structure, circle. The chemical structures of fats and oils generally look like this: Includes kit list and safety instructions. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). Devise an experimental procedure to test the chemical properties. Method Of Making Soap With Chemical Equation.
From www.c6beauty.com
Soap rancidity C6 Beauty Method Of Making Soap With Chemical Equation Devise an experimental procedure to test the chemical properties of soap. You will precipitate the soap by adding it to a. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together. Method Of Making Soap With Chemical Equation.
From www.youtube.com
What is Saponification? Structure and Action of Soaps and Detergents Method Of Making Soap With Chemical Equation You will precipitate the soap by adding it to a. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). Try this class practical to make a soap or detergent using castor oil and either sodium hydroxide or sulfuric acid. Devise an experimental procedure to test the chemical properties. Method Of Making Soap With Chemical Equation.
From spmchemistry.blog.onlinetuition.com.my
The Making of Soap Saponification SPM Chemistry Method Of Making Soap With Chemical Equation Explain why soap has the ability to clean greasy surfaces or stains. The chemical formula of the soap is ch3(ch2)14coona+. Includes kit list and safety instructions. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). One of the organic chemical reactions known to ancient man was the preparation. Method Of Making Soap With Chemical Equation.
From stock.adobe.com
Saponification equation, reaction of soap, chemistry equation of soap Method Of Making Soap With Chemical Equation You will precipitate the soap by adding it to a. The chemical formula of the soap is ch3(ch2)14coona+. Devise an experimental procedure to test the chemical properties of soap. Includes kit list and safety instructions. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). Natural soaps are sodium. Method Of Making Soap With Chemical Equation.
From www.numerade.com
SOLVED Soaps are produced by the reaction of sodium hydroxide with Method Of Making Soap With Chemical Equation Explain why soap has the ability to clean greasy surfaces or stains. Try this class practical to make a soap or detergent using castor oil and either sodium hydroxide or sulfuric acid. One the above structure, circle. The chemical formula of the soap is ch3(ch2)14coona+. You will precipitate the soap by adding it to a. Includes kit list and safety. Method Of Making Soap With Chemical Equation.
From in.pinterest.com
Hand washing with soap vector illustration. Educational explanation Method Of Making Soap With Chemical Equation In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. The chemical formula of the soap is ch3(ch2)14coona+. The chemical structures of fats and oils generally look like this: Includes kit list and safety instructions. One of the organic chemical reactions known to ancient man was the preparation of soaps through. Method Of Making Soap With Chemical Equation.
From www.alamy.com
General formula of solid and liquid soap molecule. RCOONa, RCOOK Method Of Making Soap With Chemical Equation The chemical formula of the soap is ch3(ch2)14coona+. One the above structure, circle. Explain why soap has the ability to clean greasy surfaces or stains. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. You will precipitate the soap by adding it to a. In this experiment, you will. Method Of Making Soap With Chemical Equation.
From hxewhpfvt.blob.core.windows.net
Soap Making Equation at Edna Fields blog Method Of Making Soap With Chemical Equation One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. You will precipitate the soap by adding it to a. The chemical formula of the soap is ch3(ch2)14coona+. Devise an experimental procedure to test the chemical properties of soap. Soap is made from reacting a fat or oil (or a. Method Of Making Soap With Chemical Equation.
From historymeetsscience.blogspot.com
Tales of scientific journeys Soap making 101 Method Of Making Soap With Chemical Equation Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye or potash (potassium hydroxide). Try this class practical to make a soap or detergent using castor oil and either sodium hydroxide or sulfuric acid. Soap is made from reacting a fat or oil (or a mixture) with a. Method Of Making Soap With Chemical Equation.
From spmchemistry.blog.onlinetuition.com.my
The Making of Detergent SPM Chemistry Method Of Making Soap With Chemical Equation Explain why soap has the ability to clean greasy surfaces or stains. You will precipitate the soap by adding it to a. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Devise an experimental procedure to test the chemical properties of soap. One the above structure, circle. In this. Method Of Making Soap With Chemical Equation.
From busy.org
Saponification 1/2 Method Of Making Soap With Chemical Equation You will precipitate the soap by adding it to a. Includes kit list and safety instructions. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. The chemical formula of the soap is ch3(ch2)14coona+. Circle the portion of the molecule that. One the above structure, circle. In this experiment, you. Method Of Making Soap With Chemical Equation.