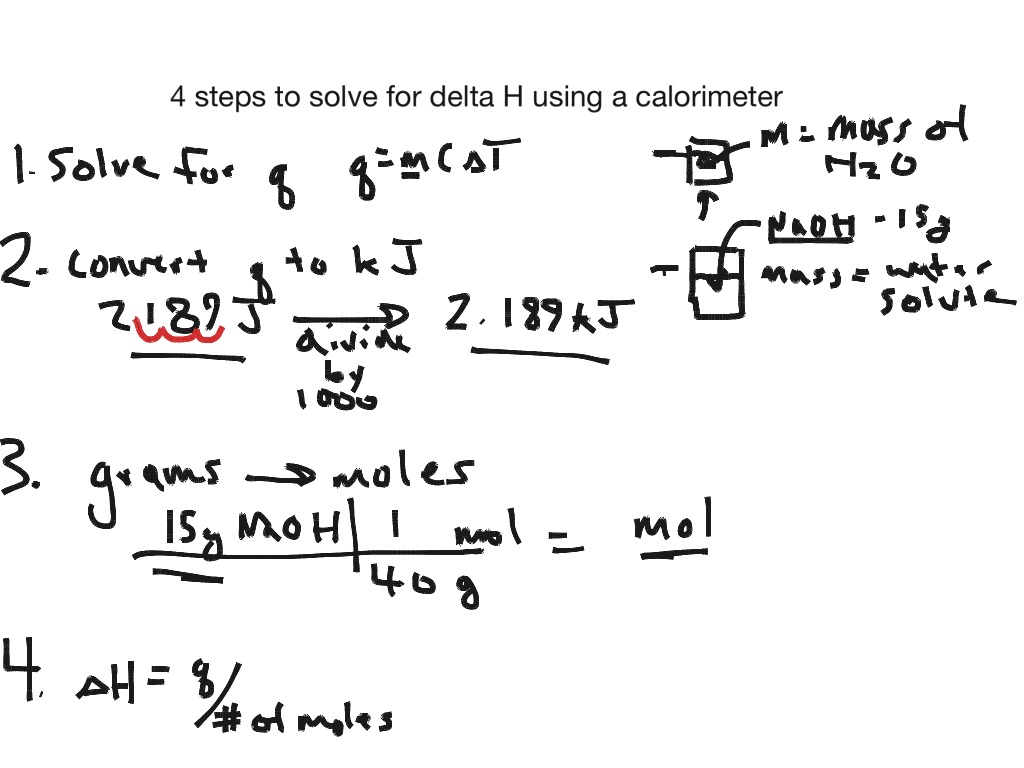
Formula For Final Temperature In Calorimetry . How to calculate final temperature of an object after heat added. Calculating the final temperature in calorimetry. In a calorimeter, we can measure temperature change with a thermometer. For the object in question, identify its mass m, specific heat c, and initial temperature ti as well as how much heat q. Apply the first law of thermodynamics to calorimetry. Consequently, you can use this information to write the following equation: This equation binds temperature change and heat: This chemistry video tutorial explains how to find the final temperature in common heat transfer. \scriptsize \delta q = m \cdot c \cdot. In calorimetry, the final temperature can be calculated using the formula: Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Reaction enthalpy = (heat capacity of contents) x (mass of. The letter q is the heat transferred in an exchange in calories, m is the mass of the substance being heated in grams, c is its.
from worksheetmagicgatewood.z13.web.core.windows.net
Reaction enthalpy = (heat capacity of contents) x (mass of. This equation binds temperature change and heat: Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. How to calculate final temperature of an object after heat added. This chemistry video tutorial explains how to find the final temperature in common heat transfer. Consequently, you can use this information to write the following equation: The letter q is the heat transferred in an exchange in calories, m is the mass of the substance being heated in grams, c is its. Apply the first law of thermodynamics to calorimetry. In a calorimeter, we can measure temperature change with a thermometer. Calculating the final temperature in calorimetry.
How To Calculate Calorimetry Problems
Formula For Final Temperature In Calorimetry This equation binds temperature change and heat: Reaction enthalpy = (heat capacity of contents) x (mass of. Consequently, you can use this information to write the following equation: For the object in question, identify its mass m, specific heat c, and initial temperature ti as well as how much heat q. In a calorimeter, we can measure temperature change with a thermometer. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Apply the first law of thermodynamics to calorimetry. This equation binds temperature change and heat: \scriptsize \delta q = m \cdot c \cdot. The letter q is the heat transferred in an exchange in calories, m is the mass of the substance being heated in grams, c is its. Calculating the final temperature in calorimetry. This chemistry video tutorial explains how to find the final temperature in common heat transfer. In calorimetry, the final temperature can be calculated using the formula: How to calculate final temperature of an object after heat added.
From www.youtube.com
Temperature and Heat Calorimetry. Level 2, Example 2 YouTube Formula For Final Temperature In Calorimetry Apply the first law of thermodynamics to calorimetry. Reaction enthalpy = (heat capacity of contents) x (mass of. In a calorimeter, we can measure temperature change with a thermometer. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. For the object in question, identify its mass m, specific heat c, and initial temperature. Formula For Final Temperature In Calorimetry.
From www.slideserve.com
PPT Gases and Temperature Changes PowerPoint Presentation, free download ID6548817 Formula For Final Temperature In Calorimetry The letter q is the heat transferred in an exchange in calories, m is the mass of the substance being heated in grams, c is its. How to calculate final temperature of an object after heat added. Calculating the final temperature in calorimetry. For the object in question, identify its mass m, specific heat c, and initial temperature ti as. Formula For Final Temperature In Calorimetry.
From www.youtube.com
Charles's Law Solving for Final Temperature YouTube Formula For Final Temperature In Calorimetry Calculating the final temperature in calorimetry. Reaction enthalpy = (heat capacity of contents) x (mass of. This chemistry video tutorial explains how to find the final temperature in common heat transfer. Consequently, you can use this information to write the following equation: The letter q is the heat transferred in an exchange in calories, m is the mass of the. Formula For Final Temperature In Calorimetry.
From www.slideserve.com
PPT HEAT EQUATION (in Table T) PowerPoint Presentation, free download ID3203245 Formula For Final Temperature In Calorimetry How to calculate final temperature of an object after heat added. This chemistry video tutorial explains how to find the final temperature in common heat transfer. For the object in question, identify its mass m, specific heat c, and initial temperature ti as well as how much heat q. Consequently, you can use this information to write the following equation:. Formula For Final Temperature In Calorimetry.
From worksheetmediadwaum.z14.web.core.windows.net
How To Calculate Calorimetry Problems Formula For Final Temperature In Calorimetry Consequently, you can use this information to write the following equation: In calorimetry, the final temperature can be calculated using the formula: Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. This chemistry video tutorial explains how to find the final temperature in common heat. Formula For Final Temperature In Calorimetry.
From quizmischances.z4.web.core.windows.net
How To Calculate Calorimetry Formula For Final Temperature In Calorimetry Consequently, you can use this information to write the following equation: \scriptsize \delta q = m \cdot c \cdot. This equation binds temperature change and heat: This chemistry video tutorial explains how to find the final temperature in common heat transfer. Reaction enthalpy = (heat capacity of contents) x (mass of. In calorimetry, the final temperature can be calculated using. Formula For Final Temperature In Calorimetry.
From www.tessshebaylo.com
Calorimeter Equation Final Temperature Tessshebaylo Formula For Final Temperature In Calorimetry In calorimetry, the final temperature can be calculated using the formula: In a calorimeter, we can measure temperature change with a thermometer. Calculating the final temperature in calorimetry. The letter q is the heat transferred in an exchange in calories, m is the mass of the substance being heated in grams, c is its. \scriptsize \delta q = m \cdot. Formula For Final Temperature In Calorimetry.
From www.vrogue.co
Heat Temperature Formulas Examples How To Calculate T vrogue.co Formula For Final Temperature In Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Reaction enthalpy = (heat capacity of contents) x (mass of. Consequently, you can use this information to write the following equation: This chemistry video tutorial explains how to find the final temperature in common heat transfer. In calorimetry, the final temperature can be calculated. Formula For Final Temperature In Calorimetry.
From www.youtube.com
Final Temperature Calorimetry Practice Problems Chemistry YouTube Formula For Final Temperature In Calorimetry The letter q is the heat transferred in an exchange in calories, m is the mass of the substance being heated in grams, c is its. Consequently, you can use this information to write the following equation: Calculating the final temperature in calorimetry. In a calorimeter, we can measure temperature change with a thermometer. This equation binds temperature change and. Formula For Final Temperature In Calorimetry.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube Formula For Final Temperature In Calorimetry In calorimetry, the final temperature can be calculated using the formula: How to calculate final temperature of an object after heat added. Apply the first law of thermodynamics to calorimetry. \scriptsize \delta q = m \cdot c \cdot. The letter q is the heat transferred in an exchange in calories, m is the mass of the substance being heated in. Formula For Final Temperature In Calorimetry.
From www.slideserve.com
PPT Chapter 17 PowerPoint Presentation ID1792465 Formula For Final Temperature In Calorimetry In a calorimeter, we can measure temperature change with a thermometer. The letter q is the heat transferred in an exchange in calories, m is the mass of the substance being heated in grams, c is its. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calculating the final temperature in calorimetry. Consequently,. Formula For Final Temperature In Calorimetry.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo Formula For Final Temperature In Calorimetry Apply the first law of thermodynamics to calorimetry. In calorimetry, the final temperature can be calculated using the formula: How to calculate final temperature of an object after heat added. Reaction enthalpy = (heat capacity of contents) x (mass of. The letter q is the heat transferred in an exchange in calories, m is the mass of the substance being. Formula For Final Temperature In Calorimetry.
From quizmischances.z4.web.core.windows.net
How To Calculate Calorimetry Formula For Final Temperature In Calorimetry \scriptsize \delta q = m \cdot c \cdot. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. This chemistry video tutorial explains how to find the final temperature in common heat transfer. Reaction enthalpy = (heat capacity of contents) x (mass of. In calorimetry, the final temperature can be calculated using the formula:. Formula For Final Temperature In Calorimetry.
From www.tessshebaylo.com
Calorimeter Equation Final Temperature Tessshebaylo Formula For Final Temperature In Calorimetry How to calculate final temperature of an object after heat added. This chemistry video tutorial explains how to find the final temperature in common heat transfer. In a calorimeter, we can measure temperature change with a thermometer. The letter q is the heat transferred in an exchange in calories, m is the mass of the substance being heated in grams,. Formula For Final Temperature In Calorimetry.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Formula For Final Temperature In Calorimetry For the object in question, identify its mass m, specific heat c, and initial temperature ti as well as how much heat q. How to calculate final temperature of an object after heat added. Calculating the final temperature in calorimetry. In calorimetry, the final temperature can be calculated using the formula: Consequently, you can use this information to write the. Formula For Final Temperature In Calorimetry.
From www.numerade.com
SOLVED For the Dissolution Reaction What is the correct formula to the change in temperature Formula For Final Temperature In Calorimetry In a calorimeter, we can measure temperature change with a thermometer. In calorimetry, the final temperature can be calculated using the formula: Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calculating the final temperature in calorimetry. \scriptsize \delta q = m \cdot c \cdot.. Formula For Final Temperature In Calorimetry.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download ID6591088 Formula For Final Temperature In Calorimetry This chemistry video tutorial explains how to find the final temperature in common heat transfer. In calorimetry, the final temperature can be calculated using the formula: The letter q is the heat transferred in an exchange in calories, m is the mass of the substance being heated in grams, c is its. This equation binds temperature change and heat: In. Formula For Final Temperature In Calorimetry.
From www.youtube.com
Calorimetry sample problem solving for final temperature YouTube Formula For Final Temperature In Calorimetry \scriptsize \delta q = m \cdot c \cdot. This chemistry video tutorial explains how to find the final temperature in common heat transfer. The letter q is the heat transferred in an exchange in calories, m is the mass of the substance being heated in grams, c is its. For the object in question, identify its mass m, specific heat. Formula For Final Temperature In Calorimetry.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Formula For Final Temperature In Calorimetry This equation binds temperature change and heat: Consequently, you can use this information to write the following equation: Calculating the final temperature in calorimetry. This chemistry video tutorial explains how to find the final temperature in common heat transfer. The letter q is the heat transferred in an exchange in calories, m is the mass of the substance being heated. Formula For Final Temperature In Calorimetry.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Formula For Final Temperature In Calorimetry Reaction enthalpy = (heat capacity of contents) x (mass of. In a calorimeter, we can measure temperature change with a thermometer. Apply the first law of thermodynamics to calorimetry. This equation binds temperature change and heat: \scriptsize \delta q = m \cdot c \cdot. This chemistry video tutorial explains how to find the final temperature in common heat transfer. The. Formula For Final Temperature In Calorimetry.
From goodttorials.blogspot.com
How To Find Final Temperature Without Specific Heat Formula For Final Temperature In Calorimetry The letter q is the heat transferred in an exchange in calories, m is the mass of the substance being heated in grams, c is its. This equation binds temperature change and heat: \scriptsize \delta q = m \cdot c \cdot. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Consequently, you can. Formula For Final Temperature In Calorimetry.
From learningcampusscarf.z13.web.core.windows.net
How To Calculate Heat Of Reaction Calorimetry Formula For Final Temperature In Calorimetry Calculating the final temperature in calorimetry. Apply the first law of thermodynamics to calorimetry. In calorimetry, the final temperature can be calculated using the formula: The letter q is the heat transferred in an exchange in calories, m is the mass of the substance being heated in grams, c is its. Reaction enthalpy = (heat capacity of contents) x (mass. Formula For Final Temperature In Calorimetry.
From angieadeline.blogspot.com
10+ Final Temperature Calculator AngieAdeline Formula For Final Temperature In Calorimetry Reaction enthalpy = (heat capacity of contents) x (mass of. This equation binds temperature change and heat: This chemistry video tutorial explains how to find the final temperature in common heat transfer. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. In calorimetry, the final temperature can be calculated using the formula: The. Formula For Final Temperature In Calorimetry.
From www.youtube.com
Physics Final temperature of mixture Thermal properties of matter Part 6 English YouTube Formula For Final Temperature In Calorimetry In a calorimeter, we can measure temperature change with a thermometer. This chemistry video tutorial explains how to find the final temperature in common heat transfer. This equation binds temperature change and heat: \scriptsize \delta q = m \cdot c \cdot. In calorimetry, the final temperature can be calculated using the formula: Apply the first law of thermodynamics to calorimetry.. Formula For Final Temperature In Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID6655927 Formula For Final Temperature In Calorimetry This equation binds temperature change and heat: Calculating the final temperature in calorimetry. This chemistry video tutorial explains how to find the final temperature in common heat transfer. Apply the first law of thermodynamics to calorimetry. How to calculate final temperature of an object after heat added. The letter q is the heat transferred in an exchange in calories, m. Formula For Final Temperature In Calorimetry.
From worksheetmagicgatewood.z13.web.core.windows.net
How To Calculate Calorimetry Problems Formula For Final Temperature In Calorimetry Reaction enthalpy = (heat capacity of contents) x (mass of. Consequently, you can use this information to write the following equation: Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calculating the final temperature in calorimetry. This equation binds temperature change and heat: In a. Formula For Final Temperature In Calorimetry.
From www.youtube.com
Specific Heat Solving for the Final Temperature YouTube Formula For Final Temperature In Calorimetry Reaction enthalpy = (heat capacity of contents) x (mass of. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The letter q is the heat transferred in an exchange in calories, m is the mass of the substance being heated in grams, c is its. For the object in question, identify its mass. Formula For Final Temperature In Calorimetry.
From www.tessshebaylo.com
Final Equilibrium Temperature Equation Tessshebaylo Formula For Final Temperature In Calorimetry Consequently, you can use this information to write the following equation: The letter q is the heat transferred in an exchange in calories, m is the mass of the substance being heated in grams, c is its. Calculating the final temperature in calorimetry. In calorimetry, the final temperature can be calculated using the formula: Apply the first law of thermodynamics. Formula For Final Temperature In Calorimetry.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Formula For Final Temperature In Calorimetry For the object in question, identify its mass m, specific heat c, and initial temperature ti as well as how much heat q. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The letter q is the heat transferred in an exchange in calories, m is the mass of the substance being heated. Formula For Final Temperature In Calorimetry.
From www.youtube.com
Calculating final temperature through heat exchange in thermal equilibrium YouTube Formula For Final Temperature In Calorimetry \scriptsize \delta q = m \cdot c \cdot. Apply the first law of thermodynamics to calorimetry. In calorimetry, the final temperature can be calculated using the formula: Consequently, you can use this information to write the following equation: Reaction enthalpy = (heat capacity of contents) x (mass of. For the object in question, identify its mass m, specific heat c,. Formula For Final Temperature In Calorimetry.
From studydystrophic.z4.web.core.windows.net
How To Calculate Final Temperature Formula For Final Temperature In Calorimetry How to calculate final temperature of an object after heat added. Consequently, you can use this information to write the following equation: Apply the first law of thermodynamics to calorimetry. \scriptsize \delta q = m \cdot c \cdot. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calculating the final temperature in calorimetry.. Formula For Final Temperature In Calorimetry.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Formula For Final Temperature In Calorimetry In a calorimeter, we can measure temperature change with a thermometer. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Apply the first law of thermodynamics to calorimetry. This equation binds temperature change and heat: For the object in question, identify its mass m, specific heat c, and initial temperature ti as well. Formula For Final Temperature In Calorimetry.
From www.youtube.com
What is the Final Temperature given Heat (q=mcΔT) YouTube Formula For Final Temperature In Calorimetry The letter q is the heat transferred in an exchange in calories, m is the mass of the substance being heated in grams, c is its. Reaction enthalpy = (heat capacity of contents) x (mass of. In calorimetry, the final temperature can be calculated using the formula: Apply the first law of thermodynamics to calorimetry. How to calculate final temperature. Formula For Final Temperature In Calorimetry.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Formula For Final Temperature In Calorimetry In calorimetry, the final temperature can be calculated using the formula: This equation binds temperature change and heat: For the object in question, identify its mass m, specific heat c, and initial temperature ti as well as how much heat q. \scriptsize \delta q = m \cdot c \cdot. How to calculate final temperature of an object after heat added.. Formula For Final Temperature In Calorimetry.
From learningcampusscarf.z13.web.core.windows.net
How To Calculate Heat Of Reaction Calorimetry Formula For Final Temperature In Calorimetry How to calculate final temperature of an object after heat added. \scriptsize \delta q = m \cdot c \cdot. Reaction enthalpy = (heat capacity of contents) x (mass of. In calorimetry, the final temperature can be calculated using the formula: Apply the first law of thermodynamics to calorimetry. Consequently, you can use this information to write the following equation: This. Formula For Final Temperature In Calorimetry.