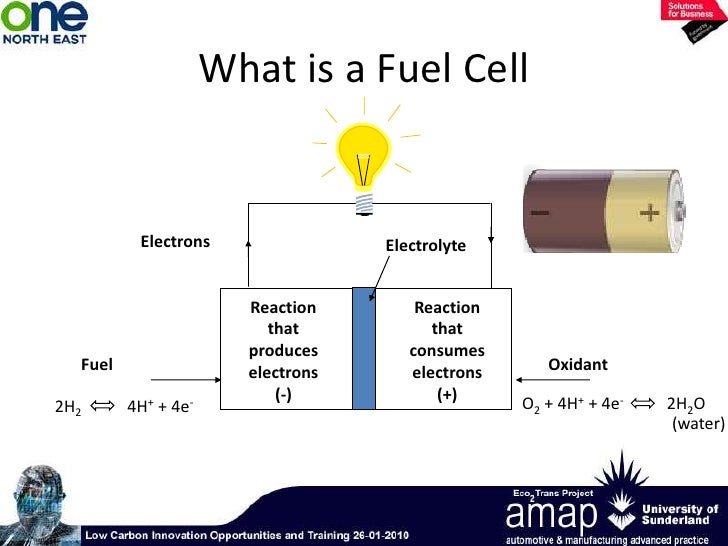
Fuel Cells Introduction . Fuel cells work like batteries, but they do not run. What they are, how they work, and what significant advantages and disadvantages they. If hydrogen is the fuel, the only. A fuel cell is an electrochemical device (a galvanic cell) which converts free energy of a chemical reaction into electrical energy. This introduction acquaints with fuel cells: A fuel cell resembles a battery in. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. A fuel cell is an electrochemical device that generates electrical energy from fuel via an electrochemical reaction. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. What is a fuel cell? Fuel cells can provide heat and electricity for buildings and electrical power for vehicles and electronic devices. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions.
from www.slideshare.net
It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. This introduction acquaints with fuel cells: Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell resembles a battery in. What is a fuel cell? Fuel cells work like batteries, but they do not run. Fuel cells can provide heat and electricity for buildings and electrical power for vehicles and electronic devices. What they are, how they work, and what significant advantages and disadvantages they. If hydrogen is the fuel, the only. A fuel cell is an electrochemical device that generates electrical energy from fuel via an electrochemical reaction.
An Introduction To Hydrogen Fuel Cells
Fuel Cells Introduction This introduction acquaints with fuel cells: Fuel cells work like batteries, but they do not run. If hydrogen is the fuel, the only. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. A fuel cell is an electrochemical device (a galvanic cell) which converts free energy of a chemical reaction into electrical energy. What is a fuel cell? Fuel cells can provide heat and electricity for buildings and electrical power for vehicles and electronic devices. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell is an electrochemical device that generates electrical energy from fuel via an electrochemical reaction. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. What they are, how they work, and what significant advantages and disadvantages they. A fuel cell resembles a battery in. This introduction acquaints with fuel cells:
From www.youtube.com
Microbial Fuel Cells Introduction (MFC's pt. 1) YouTube Fuel Cells Introduction A fuel cell resembles a battery in. What they are, how they work, and what significant advantages and disadvantages they. If hydrogen is the fuel, the only. Fuel cells work like batteries, but they do not run. A fuel cell is an electrochemical device that generates electrical energy from fuel via an electrochemical reaction. A fuel cell uses the chemical. Fuel Cells Introduction.
From www.researchgate.net
Schematic diagram of hydrogen fuel cell Download Scientific Diagram Fuel Cells Introduction A fuel cell resembles a battery in. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. What they are, how they work, and what significant advantages and disadvantages they. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. A fuel. Fuel Cells Introduction.
From www.slideserve.com
PPT Case Study Outline PowerPoint Presentation, free download ID2650163 Fuel Cells Introduction Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell is an electrochemical device that generates electrical energy from fuel via an electrochemical reaction. Fuel cells can provide heat and electricity for buildings and electrical power for vehicles and electronic devices. A fuel cell resembles. Fuel Cells Introduction.
From fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK Fuel Cells Introduction A fuel cell is an electrochemical device (a galvanic cell) which converts free energy of a chemical reaction into electrical energy. Fuel cells can provide heat and electricity for buildings and electrical power for vehicles and electronic devices. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions.. Fuel Cells Introduction.
From www.weltbild.de
Introduction to Fuel Cells Buch versandkostenfrei bei Weltbild.de Fuel Cells Introduction This introduction acquaints with fuel cells: If hydrogen is the fuel, the only. What they are, how they work, and what significant advantages and disadvantages they. What is a fuel cell? A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Fuel cells work like batteries, but they do not run. A. Fuel Cells Introduction.
From www.slideserve.com
PPT Fuel cells PowerPoint Presentation, free download ID1590103 Fuel Cells Introduction What they are, how they work, and what significant advantages and disadvantages they. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel cell is an electrochemical device (a galvanic cell) which converts free energy of a chemical reaction into electrical energy. Fuel cells work like batteries, but they do. Fuel Cells Introduction.
From www.hyseas3.eu
An introduction to Fuel Cells Fuel Cells Introduction If hydrogen is the fuel, the only. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. A fuel cell is an electrochemical device that generates electrical energy from fuel via an. Fuel Cells Introduction.
From www.britannica.com
Fuel cell Definition, Types, Applications, & Facts Britannica Fuel Cells Introduction Fuel cells can provide heat and electricity for buildings and electrical power for vehicles and electronic devices. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. What is a fuel cell? A fuel cell is an electrochemical device (a galvanic cell) which converts free energy of a. Fuel Cells Introduction.
From www.youtube.com
Introduction to Types of Fuel Cells YouTube Fuel Cells Introduction Fuel cells can provide heat and electricity for buildings and electrical power for vehicles and electronic devices. If hydrogen is the fuel, the only. A fuel cell is an electrochemical device that generates electrical energy from fuel via an electrochemical reaction. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Fuel. Fuel Cells Introduction.
From slidetodoc.com
Chapter 1 An Introduction Fuel Cells Designing and Fuel Cells Introduction What is a fuel cell? This introduction acquaints with fuel cells: What they are, how they work, and what significant advantages and disadvantages they. If hydrogen is the fuel, the only. Fuel cells can provide heat and electricity for buildings and electrical power for vehicles and electronic devices. A fuel cell uses the chemical energy of hydrogen or other fuels. Fuel Cells Introduction.
From www.nfcrc.uci.edu
National Fuel Cell Research Center (NFCRC), UC Irvine Fuel Cells Introduction A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel cell is an electrochemical device (a galvanic cell) which converts free energy of a chemical reaction into electrical energy. What they are, how they work, and what significant advantages and disadvantages they. A fuel cell resembles a battery in. It. Fuel Cells Introduction.
From www.chfca.ca
About Fuel Cells CHFCA Fuel Cells Introduction Fuel cells work like batteries, but they do not run. What is a fuel cell? Fuel cells can provide heat and electricity for buildings and electrical power for vehicles and electronic devices. What they are, how they work, and what significant advantages and disadvantages they. A fuel cell is an electrochemical device that generates electrical energy from fuel via an. Fuel Cells Introduction.
From www.youtube.com
Proton Exchange Membrane Fuel Cell, Introduction, Principle, Advantages & Disadvantages YouTube Fuel Cells Introduction This introduction acquaints with fuel cells: It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. What they are, how they work, and what significant advantages and disadvantages they. Fuel cells work like batteries, but they do not run. What is a fuel cell? A fuel cell resembles a battery in. A fuel cell. Fuel Cells Introduction.
From www.youtube.com
Microbial Fuel Cells Explained in 5 Minutes YouTube Fuel Cells Introduction A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. What is a fuel cell? It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. A fuel cell resembles a battery in. A fuel cell is an electrochemical device (a galvanic cell) which converts free energy. Fuel Cells Introduction.
From www.hyseas3.eu
An introduction to Fuel Cells Fuel Cells Introduction Fuel cells work like batteries, but they do not run. If hydrogen is the fuel, the only. A fuel cell is an electrochemical device that generates electrical energy from fuel via an electrochemical reaction. What they are, how they work, and what significant advantages and disadvantages they. Fuel cell, any of a class of devices that convert the chemical energy. Fuel Cells Introduction.
From www.researchgate.net
(PDF) INTRODUCTION TO FUEL CELL TECHNOLOGY A REVIEW Fuel Cells Introduction A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Fuel cells can provide heat and electricity for buildings and electrical power for vehicles and electronic devices. What is a fuel cell? Fuel cells work like batteries, but they do not run. What they are, how they work, and what significant advantages. Fuel Cells Introduction.
From www.slideserve.com
PPT An Introduction to Sediment Microbial Fuel Cells PowerPoint Presentation ID1737838 Fuel Cells Introduction A fuel cell resembles a battery in. Fuel cells work like batteries, but they do not run. Fuel cells can provide heat and electricity for buildings and electrical power for vehicles and electronic devices. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. This introduction acquaints with. Fuel Cells Introduction.
From large.stanford.edu
Hydrogen Fuel Cells Fuel Cells Introduction A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. This introduction acquaints with fuel cells: Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell is an electrochemical device that generates electrical energy from fuel via. Fuel Cells Introduction.
From www.youtube.com
Fuel Cell Introduction YouTube Fuel Cells Introduction A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Fuel cells can provide heat and electricity for buildings and electrical power for vehicles and electronic devices. What they are, how they work, and what significant advantages and disadvantages they. It is defined as an electrochemical cell that generates electrical energy from. Fuel Cells Introduction.
From www.slideserve.com
PPT Fuel Cell PowerPoint Presentation, free download ID4389074 Fuel Cells Introduction If hydrogen is the fuel, the only. A fuel cell resembles a battery in. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. A fuel cell is an electrochemical device that generates electrical energy from fuel via an electrochemical reaction. This introduction acquaints with fuel cells: Fuel cells can provide heat and electricity. Fuel Cells Introduction.
From www.slideshare.net
An Introduction To Hydrogen Fuel Cells Fuel Cells Introduction Fuel cells work like batteries, but they do not run. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. If hydrogen is the fuel, the only. This introduction acquaints with fuel cells: A fuel cell is an electrochemical device (a galvanic cell) which converts free energy of. Fuel Cells Introduction.
From www.slideserve.com
PPT Introduction to Fuel Cell Systems PowerPoint Presentation, free download ID3298886 Fuel Cells Introduction Fuel cells can provide heat and electricity for buildings and electrical power for vehicles and electronic devices. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cells work like batteries, but they do not run. A fuel cell resembles a battery in. What they are, how. Fuel Cells Introduction.
From www.youtube.com
Fuel Cell Introduction, Components, and Assembly YouTube Fuel Cells Introduction What they are, how they work, and what significant advantages and disadvantages they. Fuel cells can provide heat and electricity for buildings and electrical power for vehicles and electronic devices. What is a fuel cell? Fuel cells work like batteries, but they do not run. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and. Fuel Cells Introduction.
From www.slideserve.com
PPT Introduction to Fuel Cell Systems PowerPoint Presentation, free download ID3298886 Fuel Cells Introduction A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell is. Fuel Cells Introduction.
From www.slideserve.com
PPT Introduction to Fuel Cells PowerPoint Presentation, free download ID6602082 Fuel Cells Introduction If hydrogen is the fuel, the only. A fuel cell is an electrochemical device that generates electrical energy from fuel via an electrochemical reaction. Fuel cells can provide heat and electricity for buildings and electrical power for vehicles and electronic devices. A fuel cell is an electrochemical device (a galvanic cell) which converts free energy of a chemical reaction into. Fuel Cells Introduction.
From www.engineeringity.com
Breaking Down Fuel Cells How they Work and their Components The Engineeringity Fuel Cells Introduction A fuel cell resembles a battery in. This introduction acquaints with fuel cells: A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. If hydrogen is the fuel, the only. What is a fuel cell? What they are, how they work, and what significant advantages and disadvantages they. A fuel cell is. Fuel Cells Introduction.
From www.youtube.com
Fuel cell introduction and classification YouTube Fuel Cells Introduction If hydrogen is the fuel, the only. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell is an electrochemical device (a galvanic cell) which converts free. Fuel Cells Introduction.
From www.slideserve.com
PPT Fuel Cell PowerPoint Presentation, free download ID4389074 Fuel Cells Introduction Fuel cells can provide heat and electricity for buildings and electrical power for vehicles and electronic devices. A fuel cell is an electrochemical device that generates electrical energy from fuel via an electrochemical reaction. What is a fuel cell? A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Fuel cell, any. Fuel Cells Introduction.
From www.youtube.com
Phosphoric Acid Fuel Cell, Introduction, Principle, Advantages, Uses, & Disadvantages YouTube Fuel Cells Introduction A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel cell resembles a battery in. Fuel cells work like batteries, but they do not run. A fuel cell is an electrochemical device that generates electrical energy from fuel via an electrochemical reaction. Fuel cell, any of a class of devices. Fuel Cells Introduction.
From www.youtube.com
Direct Methanol Fuel Cell, Introduction, Principle, Advantages, Disadvantages & Uses YouTube Fuel Cells Introduction A fuel cell resembles a battery in. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. This introduction acquaints with fuel cells: If hydrogen is the fuel, the only. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. Fuel cells work like batteries, but. Fuel Cells Introduction.
From www.e-conversion.de
Fuel cell principle econversion Fuel Cells Introduction A fuel cell is an electrochemical device that generates electrical energy from fuel via an electrochemical reaction. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cells work. Fuel Cells Introduction.
From www.britannica.com
Fuel cell Definition, Types, Applications, & Facts Britannica Fuel Cells Introduction If hydrogen is the fuel, the only. A fuel cell resembles a battery in. This introduction acquaints with fuel cells: What is a fuel cell? A fuel cell is an electrochemical device that generates electrical energy from fuel via an electrochemical reaction. What they are, how they work, and what significant advantages and disadvantages they. Fuel cells work like batteries,. Fuel Cells Introduction.
From edfuturetech.com
Fuel Cells 101 An Introduction to the Basics of Fuel Cell Technology Education in Future Fuel Cells Introduction Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cells work like batteries, but they do not run. A fuel cell resembles a battery in. A fuel cell is an electrochemical device that generates electrical energy from fuel via an electrochemical reaction. This introduction acquaints with. Fuel Cells Introduction.
From www.slideserve.com
PPT FUEL CELLS PowerPoint Presentation, free download ID752738 Fuel Cells Introduction It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. If hydrogen is the fuel, the only. Fuel cells work like batteries, but they do not run. A fuel cell is an electrochemical device (a galvanic. Fuel Cells Introduction.
From www.researchgate.net
e Schematic view of a typical hydrogen PEM fuel cell and its components. Download Scientific Fuel Cells Introduction A fuel cell resembles a battery in. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. This introduction acquaints with fuel cells: A fuel cell is an electrochemical device (a galvanic cell) which converts free energy of a chemical reaction into electrical energy. It is defined as. Fuel Cells Introduction.