How Much Power Is Needed For Electrolysis . Electrochemistry is the perfect fusion of physics and chemistry: The model determines cell voltage and specific load and calculates the power requirements for the electrolysis reaction. I use 12v at 2a. Electrolysis is the process of using electricity to split water into hydrogen and. This added voltage, called an overvoltage, represents the additional. Solve your electrochemical problems with our faraday's law of electrolysis calculator! I'm using naoh for the electrolyte. Approximately 1 tablespoon per gallon with 15 gallons. Sep 18, 2015 at 3:36. The 237kj/mol is the gibbs free energy required for electrolysis, while the 286kj/mol normally quoted for combustion is the enthalpy.
from educationforyoursuccess.blogspot.com
Sep 18, 2015 at 3:36. The model determines cell voltage and specific load and calculates the power requirements for the electrolysis reaction. The 237kj/mol is the gibbs free energy required for electrolysis, while the 286kj/mol normally quoted for combustion is the enthalpy. I use 12v at 2a. Solve your electrochemical problems with our faraday's law of electrolysis calculator! Electrolysis is the process of using electricity to split water into hydrogen and. This added voltage, called an overvoltage, represents the additional. Electrochemistry is the perfect fusion of physics and chemistry: Approximately 1 tablespoon per gallon with 15 gallons. I'm using naoh for the electrolyte.
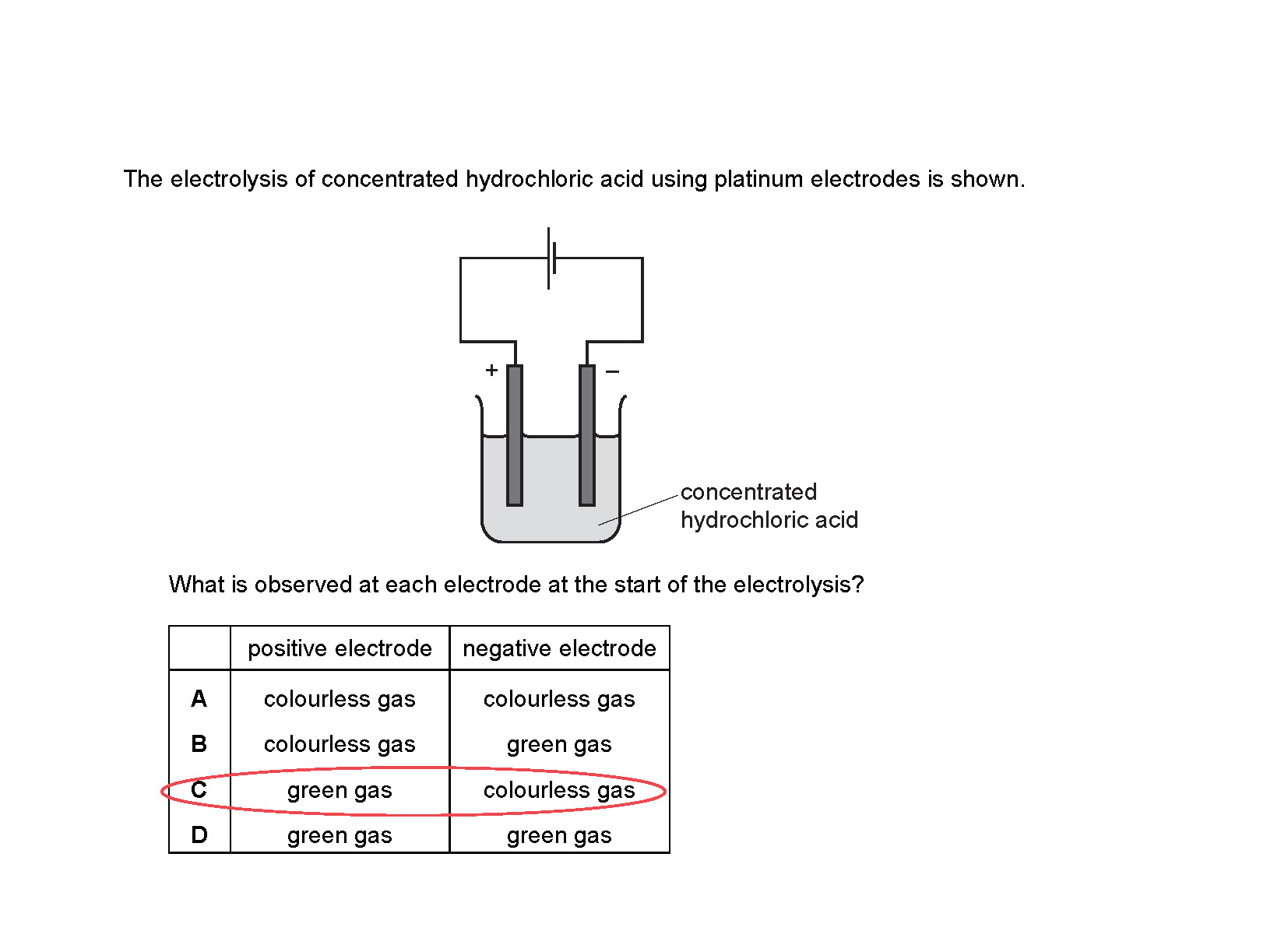
Electrolysis (Electricity & Chemistry) IGCSE 0620 / O Levels Paper 2 Solved MCQs
How Much Power Is Needed For Electrolysis I use 12v at 2a. Sep 18, 2015 at 3:36. Electrochemistry is the perfect fusion of physics and chemistry: Solve your electrochemical problems with our faraday's law of electrolysis calculator! Electrolysis is the process of using electricity to split water into hydrogen and. The model determines cell voltage and specific load and calculates the power requirements for the electrolysis reaction. This added voltage, called an overvoltage, represents the additional. The 237kj/mol is the gibbs free energy required for electrolysis, while the 286kj/mol normally quoted for combustion is the enthalpy. I'm using naoh for the electrolyte. Approximately 1 tablespoon per gallon with 15 gallons. I use 12v at 2a.
From app.pandai.org
Electrolytic Cell How Much Power Is Needed For Electrolysis The model determines cell voltage and specific load and calculates the power requirements for the electrolysis reaction. I'm using naoh for the electrolyte. Electrochemistry is the perfect fusion of physics and chemistry: Electrolysis is the process of using electricity to split water into hydrogen and. I use 12v at 2a. Approximately 1 tablespoon per gallon with 15 gallons. Sep 18,. How Much Power Is Needed For Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk How Much Power Is Needed For Electrolysis Sep 18, 2015 at 3:36. The model determines cell voltage and specific load and calculates the power requirements for the electrolysis reaction. Electrolysis is the process of using electricity to split water into hydrogen and. This added voltage, called an overvoltage, represents the additional. Approximately 1 tablespoon per gallon with 15 gallons. Solve your electrochemical problems with our faraday's law. How Much Power Is Needed For Electrolysis.
From byjus.com
Water Electrolysis Principle of Water Electrolysis, Important Factors, Electrolytes How Much Power Is Needed For Electrolysis Electrolysis is the process of using electricity to split water into hydrogen and. The 237kj/mol is the gibbs free energy required for electrolysis, while the 286kj/mol normally quoted for combustion is the enthalpy. This added voltage, called an overvoltage, represents the additional. Electrochemistry is the perfect fusion of physics and chemistry: I'm using naoh for the electrolyte. I use 12v. How Much Power Is Needed For Electrolysis.
From phys.org
A powerful catalyst for electrolysis of water that could help harness renewable energy How Much Power Is Needed For Electrolysis I'm using naoh for the electrolyte. Approximately 1 tablespoon per gallon with 15 gallons. This added voltage, called an overvoltage, represents the additional. I use 12v at 2a. The 237kj/mol is the gibbs free energy required for electrolysis, while the 286kj/mol normally quoted for combustion is the enthalpy. Solve your electrochemical problems with our faraday's law of electrolysis calculator! Sep. How Much Power Is Needed For Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk How Much Power Is Needed For Electrolysis I'm using naoh for the electrolyte. Solve your electrochemical problems with our faraday's law of electrolysis calculator! This added voltage, called an overvoltage, represents the additional. The 237kj/mol is the gibbs free energy required for electrolysis, while the 286kj/mol normally quoted for combustion is the enthalpy. The model determines cell voltage and specific load and calculates the power requirements for. How Much Power Is Needed For Electrolysis.
From study.com
Faraday's Laws of Electrolysis Equation & Constant Units Lesson How Much Power Is Needed For Electrolysis Solve your electrochemical problems with our faraday's law of electrolysis calculator! The model determines cell voltage and specific load and calculates the power requirements for the electrolysis reaction. Electrochemistry is the perfect fusion of physics and chemistry: This added voltage, called an overvoltage, represents the additional. I use 12v at 2a. The 237kj/mol is the gibbs free energy required for. How Much Power Is Needed For Electrolysis.
From www.oceangeothermal.org
Hydrogen Through Electrolysis Ocean Geothermal Energy Foundation How Much Power Is Needed For Electrolysis Approximately 1 tablespoon per gallon with 15 gallons. Sep 18, 2015 at 3:36. Solve your electrochemical problems with our faraday's law of electrolysis calculator! I use 12v at 2a. Electrochemistry is the perfect fusion of physics and chemistry: The 237kj/mol is the gibbs free energy required for electrolysis, while the 286kj/mol normally quoted for combustion is the enthalpy. Electrolysis is. How Much Power Is Needed For Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID3366458 How Much Power Is Needed For Electrolysis I use 12v at 2a. This added voltage, called an overvoltage, represents the additional. The 237kj/mol is the gibbs free energy required for electrolysis, while the 286kj/mol normally quoted for combustion is the enthalpy. The model determines cell voltage and specific load and calculates the power requirements for the electrolysis reaction. Solve your electrochemical problems with our faraday's law of. How Much Power Is Needed For Electrolysis.
From www.youtube.com
Experiment How to make a device for electrolysis at home YouTube How Much Power Is Needed For Electrolysis Electrochemistry is the perfect fusion of physics and chemistry: Solve your electrochemical problems with our faraday's law of electrolysis calculator! I'm using naoh for the electrolyte. I use 12v at 2a. The 237kj/mol is the gibbs free energy required for electrolysis, while the 286kj/mol normally quoted for combustion is the enthalpy. The model determines cell voltage and specific load and. How Much Power Is Needed For Electrolysis.
From chem.libretexts.org
5.6 Day 41 Electrolysis; Commercial Batteries Chemistry LibreTexts How Much Power Is Needed For Electrolysis Electrolysis is the process of using electricity to split water into hydrogen and. I'm using naoh for the electrolyte. The 237kj/mol is the gibbs free energy required for electrolysis, while the 286kj/mol normally quoted for combustion is the enthalpy. The model determines cell voltage and specific load and calculates the power requirements for the electrolysis reaction. I use 12v at. How Much Power Is Needed For Electrolysis.
From www.youtube.com
Electrolysis of Salt Solution at Home in Under 1 minute to produce Hydrogen and Chlorine YouTube How Much Power Is Needed For Electrolysis I'm using naoh for the electrolyte. Solve your electrochemical problems with our faraday's law of electrolysis calculator! Sep 18, 2015 at 3:36. Approximately 1 tablespoon per gallon with 15 gallons. The model determines cell voltage and specific load and calculates the power requirements for the electrolysis reaction. I use 12v at 2a. Electrolysis is the process of using electricity to. How Much Power Is Needed For Electrolysis.
From www.toppr.com
How much electricity in terms of Faraday is required to produce(i) 20 g of Ca from molten CaCl2 How Much Power Is Needed For Electrolysis Electrolysis is the process of using electricity to split water into hydrogen and. I'm using naoh for the electrolyte. Approximately 1 tablespoon per gallon with 15 gallons. Sep 18, 2015 at 3:36. The model determines cell voltage and specific load and calculates the power requirements for the electrolysis reaction. I use 12v at 2a. Solve your electrochemical problems with our. How Much Power Is Needed For Electrolysis.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace How Much Power Is Needed For Electrolysis The model determines cell voltage and specific load and calculates the power requirements for the electrolysis reaction. I use 12v at 2a. Approximately 1 tablespoon per gallon with 15 gallons. Electrolysis is the process of using electricity to split water into hydrogen and. Solve your electrochemical problems with our faraday's law of electrolysis calculator! Sep 18, 2015 at 3:36. The. How Much Power Is Needed For Electrolysis.
From energy.gov
Hydrogen Production Electrolysis Department of Energy How Much Power Is Needed For Electrolysis The model determines cell voltage and specific load and calculates the power requirements for the electrolysis reaction. Approximately 1 tablespoon per gallon with 15 gallons. Solve your electrochemical problems with our faraday's law of electrolysis calculator! I use 12v at 2a. This added voltage, called an overvoltage, represents the additional. Sep 18, 2015 at 3:36. I'm using naoh for the. How Much Power Is Needed For Electrolysis.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes PtX Hub How Much Power Is Needed For Electrolysis Approximately 1 tablespoon per gallon with 15 gallons. Electrochemistry is the perfect fusion of physics and chemistry: This added voltage, called an overvoltage, represents the additional. Electrolysis is the process of using electricity to split water into hydrogen and. Solve your electrochemical problems with our faraday's law of electrolysis calculator! Sep 18, 2015 at 3:36. I use 12v at 2a.. How Much Power Is Needed For Electrolysis.
From mungfali.com
Electrolysis Process Diagram How Much Power Is Needed For Electrolysis The 237kj/mol is the gibbs free energy required for electrolysis, while the 286kj/mol normally quoted for combustion is the enthalpy. Approximately 1 tablespoon per gallon with 15 gallons. Electrolysis is the process of using electricity to split water into hydrogen and. Solve your electrochemical problems with our faraday's law of electrolysis calculator! The model determines cell voltage and specific load. How Much Power Is Needed For Electrolysis.
From study.com
Electrolysis of Aqueous Solutions Lesson How Much Power Is Needed For Electrolysis The model determines cell voltage and specific load and calculates the power requirements for the electrolysis reaction. Sep 18, 2015 at 3:36. Electrochemistry is the perfect fusion of physics and chemistry: Electrolysis is the process of using electricity to split water into hydrogen and. I use 12v at 2a. Approximately 1 tablespoon per gallon with 15 gallons. Solve your electrochemical. How Much Power Is Needed For Electrolysis.
From www.youtube.com
WATER ELECTROLYSIS DEMONSTRATION WITH EXPLANATION CHEMISTRY GRADE 812 YouTube How Much Power Is Needed For Electrolysis The 237kj/mol is the gibbs free energy required for electrolysis, while the 286kj/mol normally quoted for combustion is the enthalpy. I'm using naoh for the electrolyte. This added voltage, called an overvoltage, represents the additional. Electrolysis is the process of using electricity to split water into hydrogen and. Sep 18, 2015 at 3:36. Approximately 1 tablespoon per gallon with 15. How Much Power Is Needed For Electrolysis.
From www.researchgate.net
The currentvoltage performances of a single PEM electrolysis cell... Download Scientific Diagram How Much Power Is Needed For Electrolysis Sep 18, 2015 at 3:36. Electrolysis is the process of using electricity to split water into hydrogen and. The 237kj/mol is the gibbs free energy required for electrolysis, while the 286kj/mol normally quoted for combustion is the enthalpy. The model determines cell voltage and specific load and calculates the power requirements for the electrolysis reaction. I use 12v at 2a.. How Much Power Is Needed For Electrolysis.
From www.youtube.com
Using Electrolysis To Extract Metals (GCSE Chemistry) YouTube How Much Power Is Needed For Electrolysis Approximately 1 tablespoon per gallon with 15 gallons. The 237kj/mol is the gibbs free energy required for electrolysis, while the 286kj/mol normally quoted for combustion is the enthalpy. This added voltage, called an overvoltage, represents the additional. I use 12v at 2a. Electrochemistry is the perfect fusion of physics and chemistry: The model determines cell voltage and specific load and. How Much Power Is Needed For Electrolysis.
From www.alamy.com
Electroplating with copper using copper sulfate electrolyte. Electrolysis of copper(II) sulfate How Much Power Is Needed For Electrolysis I'm using naoh for the electrolyte. Electrolysis is the process of using electricity to split water into hydrogen and. Approximately 1 tablespoon per gallon with 15 gallons. Sep 18, 2015 at 3:36. The 237kj/mol is the gibbs free energy required for electrolysis, while the 286kj/mol normally quoted for combustion is the enthalpy. The model determines cell voltage and specific load. How Much Power Is Needed For Electrolysis.
From www.pinterest.com
Electrolysis Chemical changes, Electrochemistry, Energy How Much Power Is Needed For Electrolysis Approximately 1 tablespoon per gallon with 15 gallons. This added voltage, called an overvoltage, represents the additional. The model determines cell voltage and specific load and calculates the power requirements for the electrolysis reaction. I'm using naoh for the electrolyte. I use 12v at 2a. Electrochemistry is the perfect fusion of physics and chemistry: The 237kj/mol is the gibbs free. How Much Power Is Needed For Electrolysis.
From general.chemistrysteps.com
Electrolysis Chemistry Steps How Much Power Is Needed For Electrolysis I'm using naoh for the electrolyte. The model determines cell voltage and specific load and calculates the power requirements for the electrolysis reaction. Electrolysis is the process of using electricity to split water into hydrogen and. This added voltage, called an overvoltage, represents the additional. I use 12v at 2a. Electrochemistry is the perfect fusion of physics and chemistry: The. How Much Power Is Needed For Electrolysis.
From en.ppt-online.org
Electrolysis online presentation How Much Power Is Needed For Electrolysis I'm using naoh for the electrolyte. Sep 18, 2015 at 3:36. The model determines cell voltage and specific load and calculates the power requirements for the electrolysis reaction. I use 12v at 2a. Approximately 1 tablespoon per gallon with 15 gallons. Solve your electrochemical problems with our faraday's law of electrolysis calculator! Electrochemistry is the perfect fusion of physics and. How Much Power Is Needed For Electrolysis.
From www.awoe.net
A World Of Energy Water electrolysis How Much Power Is Needed For Electrolysis I'm using naoh for the electrolyte. This added voltage, called an overvoltage, represents the additional. Electrochemistry is the perfect fusion of physics and chemistry: The 237kj/mol is the gibbs free energy required for electrolysis, while the 286kj/mol normally quoted for combustion is the enthalpy. Sep 18, 2015 at 3:36. The model determines cell voltage and specific load and calculates the. How Much Power Is Needed For Electrolysis.
From mungfali.com
Electrolysis Of Sodium Chloride Diagram How Much Power Is Needed For Electrolysis Solve your electrochemical problems with our faraday's law of electrolysis calculator! I'm using naoh for the electrolyte. This added voltage, called an overvoltage, represents the additional. The 237kj/mol is the gibbs free energy required for electrolysis, while the 286kj/mol normally quoted for combustion is the enthalpy. Electrochemistry is the perfect fusion of physics and chemistry: The model determines cell voltage. How Much Power Is Needed For Electrolysis.
From educationforyoursuccess.blogspot.com
Electrolysis (Electricity & Chemistry) IGCSE 0620 / O Levels Paper 2 Solved MCQs How Much Power Is Needed For Electrolysis I'm using naoh for the electrolyte. This added voltage, called an overvoltage, represents the additional. I use 12v at 2a. Approximately 1 tablespoon per gallon with 15 gallons. The 237kj/mol is the gibbs free energy required for electrolysis, while the 286kj/mol normally quoted for combustion is the enthalpy. Electrochemistry is the perfect fusion of physics and chemistry: Sep 18, 2015. How Much Power Is Needed For Electrolysis.
From www.toppr.com
How much electricity in terms of Faraday is required to produce 100 g of Ca from molten CaCl2 How Much Power Is Needed For Electrolysis This added voltage, called an overvoltage, represents the additional. I use 12v at 2a. Sep 18, 2015 at 3:36. The 237kj/mol is the gibbs free energy required for electrolysis, while the 286kj/mol normally quoted for combustion is the enthalpy. I'm using naoh for the electrolyte. Solve your electrochemical problems with our faraday's law of electrolysis calculator! The model determines cell. How Much Power Is Needed For Electrolysis.
From www.researchgate.net
Schematic diagram of electrolysis process (1) DC power supply, (2)... Download Scientific Diagram How Much Power Is Needed For Electrolysis I use 12v at 2a. Sep 18, 2015 at 3:36. The 237kj/mol is the gibbs free energy required for electrolysis, while the 286kj/mol normally quoted for combustion is the enthalpy. Electrochemistry is the perfect fusion of physics and chemistry: Electrolysis is the process of using electricity to split water into hydrogen and. Approximately 1 tablespoon per gallon with 15 gallons.. How Much Power Is Needed For Electrolysis.
From www.freepik.com
Free Vector Electrolysis diagram experiment for education How Much Power Is Needed For Electrolysis Electrochemistry is the perfect fusion of physics and chemistry: Solve your electrochemical problems with our faraday's law of electrolysis calculator! Approximately 1 tablespoon per gallon with 15 gallons. The model determines cell voltage and specific load and calculates the power requirements for the electrolysis reaction. I use 12v at 2a. This added voltage, called an overvoltage, represents the additional. I'm. How Much Power Is Needed For Electrolysis.
From socratic.org
Electrolysis Chemistry Socratic How Much Power Is Needed For Electrolysis I'm using naoh for the electrolyte. Approximately 1 tablespoon per gallon with 15 gallons. Electrochemistry is the perfect fusion of physics and chemistry: Sep 18, 2015 at 3:36. The 237kj/mol is the gibbs free energy required for electrolysis, while the 286kj/mol normally quoted for combustion is the enthalpy. Solve your electrochemical problems with our faraday's law of electrolysis calculator! Electrolysis. How Much Power Is Needed For Electrolysis.
From byjus.com
Conduction of Electricity in Liquids Electrolysis, Reduction at cathode, Oxidation at Anode How Much Power Is Needed For Electrolysis Solve your electrochemical problems with our faraday's law of electrolysis calculator! Sep 18, 2015 at 3:36. This added voltage, called an overvoltage, represents the additional. Electrolysis is the process of using electricity to split water into hydrogen and. The 237kj/mol is the gibbs free energy required for electrolysis, while the 286kj/mol normally quoted for combustion is the enthalpy. Electrochemistry is. How Much Power Is Needed For Electrolysis.
From byjus.com
Does Electrolysis produce electricity? How Much Power Is Needed For Electrolysis This added voltage, called an overvoltage, represents the additional. Electrochemistry is the perfect fusion of physics and chemistry: Approximately 1 tablespoon per gallon with 15 gallons. The model determines cell voltage and specific load and calculates the power requirements for the electrolysis reaction. I use 12v at 2a. I'm using naoh for the electrolyte. Sep 18, 2015 at 3:36. Electrolysis. How Much Power Is Needed For Electrolysis.
From www.freepik.com
Premium Vector Electrolysis process vector illustration diagram How Much Power Is Needed For Electrolysis Electrolysis is the process of using electricity to split water into hydrogen and. This added voltage, called an overvoltage, represents the additional. Sep 18, 2015 at 3:36. Approximately 1 tablespoon per gallon with 15 gallons. I'm using naoh for the electrolyte. I use 12v at 2a. The model determines cell voltage and specific load and calculates the power requirements for. How Much Power Is Needed For Electrolysis.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts How Much Power Is Needed For Electrolysis This added voltage, called an overvoltage, represents the additional. Electrochemistry is the perfect fusion of physics and chemistry: Solve your electrochemical problems with our faraday's law of electrolysis calculator! Approximately 1 tablespoon per gallon with 15 gallons. I use 12v at 2a. Electrolysis is the process of using electricity to split water into hydrogen and. I'm using naoh for the. How Much Power Is Needed For Electrolysis.