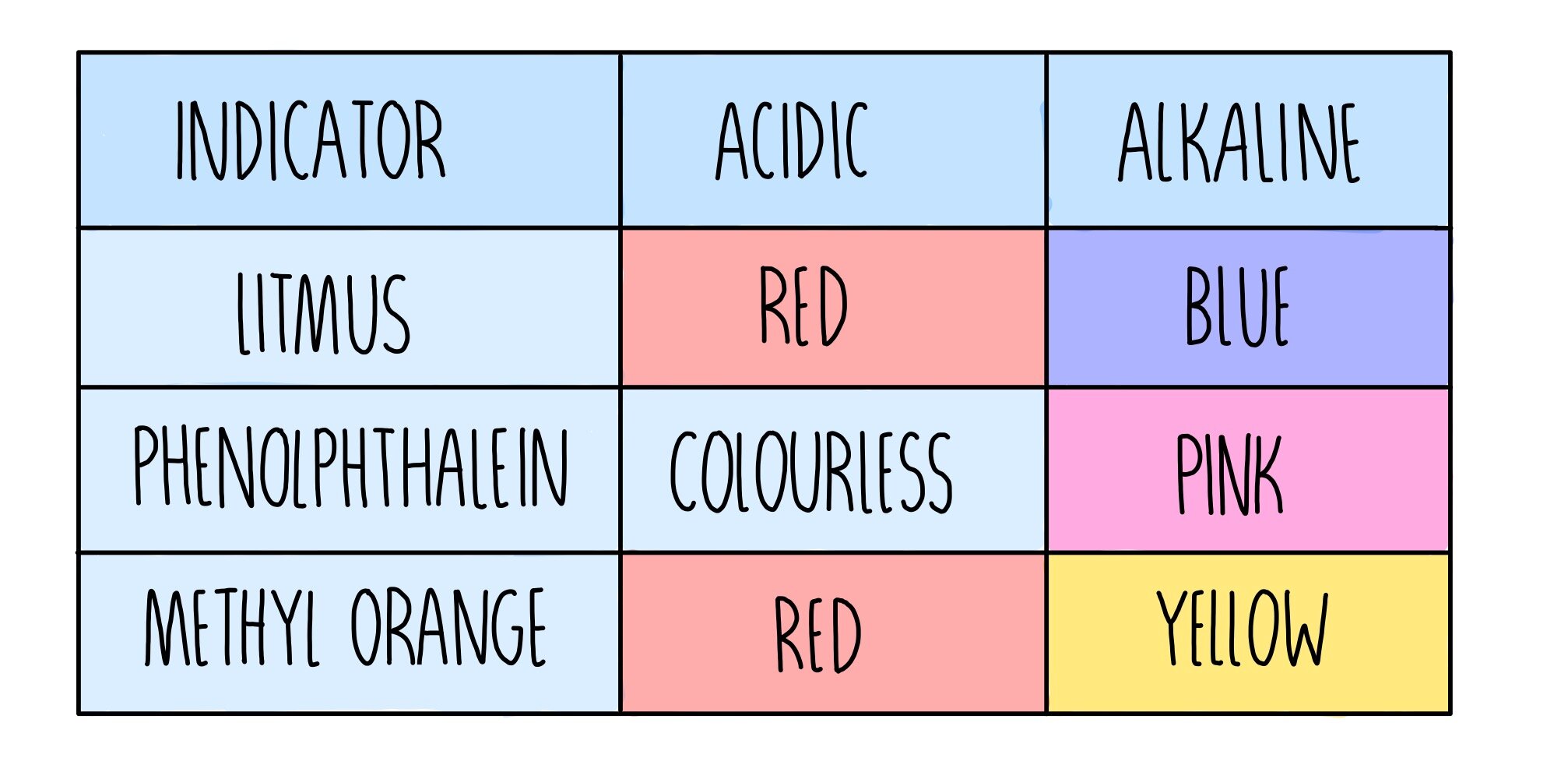
What Is An Acid Igcse . Examples of commonly known acids include. A solution that contains the maximum amount of dissolved solute at a specified temperature. A neutralisation reaction occurs when an acid reacts with an alkali. A series of free igcse chemistry lessons (cambridge igcse chemistry). Study with quizlet and memorise flashcards. Define a strong acid as an acid that is completely dissociated in aqueous solution and. An acid is a compound which when dissolved in water produces hydrogen ions (h^+^) and are described as proton donors. Acids are substances that can donate a proton (h+ ion) when dissolved in water. The ph scale is a numerical scale which is used to show how acidic or alkaline a solution is. An acid is any substance that in water solution tastes sour, changes blue litmus paper to red, reacts with some metals to. Define acids as proton donors and bases as proton acceptors.
from www.thesciencehive.co.uk
Define a strong acid as an acid that is completely dissociated in aqueous solution and. Examples of commonly known acids include. A series of free igcse chemistry lessons (cambridge igcse chemistry). A neutralisation reaction occurs when an acid reacts with an alkali. An acid is a compound which when dissolved in water produces hydrogen ions (h^+^) and are described as proton donors. The ph scale is a numerical scale which is used to show how acidic or alkaline a solution is. A solution that contains the maximum amount of dissolved solute at a specified temperature. Study with quizlet and memorise flashcards. Define acids as proton donors and bases as proton acceptors. Acids are substances that can donate a proton (h+ ion) when dissolved in water.
Acids, Alkalis and Titrations (GCSE) — the science hive
What Is An Acid Igcse An acid is a compound which when dissolved in water produces hydrogen ions (h^+^) and are described as proton donors. Acids are substances that can donate a proton (h+ ion) when dissolved in water. An acid is any substance that in water solution tastes sour, changes blue litmus paper to red, reacts with some metals to. Study with quizlet and memorise flashcards. A solution that contains the maximum amount of dissolved solute at a specified temperature. Examples of commonly known acids include. A neutralisation reaction occurs when an acid reacts with an alkali. Define acids as proton donors and bases as proton acceptors. Define a strong acid as an acid that is completely dissociated in aqueous solution and. A series of free igcse chemistry lessons (cambridge igcse chemistry). An acid is a compound which when dissolved in water produces hydrogen ions (h^+^) and are described as proton donors. The ph scale is a numerical scale which is used to show how acidic or alkaline a solution is.
From www.studypool.com
SOLUTION Acid alkali and titration chemistry igcse edexcel notes 2 What Is An Acid Igcse Acids are substances that can donate a proton (h+ ion) when dissolved in water. Define acids as proton donors and bases as proton acceptors. A neutralisation reaction occurs when an acid reacts with an alkali. Define a strong acid as an acid that is completely dissociated in aqueous solution and. The ph scale is a numerical scale which is used. What Is An Acid Igcse.
From www.studypool.com
SOLUTION acid, base and salt igcse chemistry Studypool What Is An Acid Igcse Acids are substances that can donate a proton (h+ ion) when dissolved in water. Study with quizlet and memorise flashcards. A solution that contains the maximum amount of dissolved solute at a specified temperature. A series of free igcse chemistry lessons (cambridge igcse chemistry). Define a strong acid as an acid that is completely dissociated in aqueous solution and. An. What Is An Acid Igcse.
From www.youtube.com
Characteristics of Acid Cambridge IGCSE O level Chemistry 0620 0971 What Is An Acid Igcse The ph scale is a numerical scale which is used to show how acidic or alkaline a solution is. Examples of commonly known acids include. Define acids as proton donors and bases as proton acceptors. Define a strong acid as an acid that is completely dissociated in aqueous solution and. An acid is a compound which when dissolved in water. What Is An Acid Igcse.
From slideplayer.com
Quiz What is an acid? What is a base? ppt download What Is An Acid Igcse An acid is a compound which when dissolved in water produces hydrogen ions (h^+^) and are described as proton donors. Acids are substances that can donate a proton (h+ ion) when dissolved in water. A series of free igcse chemistry lessons (cambridge igcse chemistry). Define acids as proton donors and bases as proton acceptors. A solution that contains the maximum. What Is An Acid Igcse.
From www.scribd.com
Acid Bases and Salts Igcse PDF What Is An Acid Igcse Study with quizlet and memorise flashcards. Acids are substances that can donate a proton (h+ ion) when dissolved in water. A neutralisation reaction occurs when an acid reacts with an alkali. A solution that contains the maximum amount of dissolved solute at a specified temperature. A series of free igcse chemistry lessons (cambridge igcse chemistry). An acid is a compound. What Is An Acid Igcse.
From www.youtube.com
iGCSE / GCSE Chemistry How to make ethanoic acid? (14.16) YouTube What Is An Acid Igcse An acid is any substance that in water solution tastes sour, changes blue litmus paper to red, reacts with some metals to. Acids are substances that can donate a proton (h+ ion) when dissolved in water. Examples of commonly known acids include. The ph scale is a numerical scale which is used to show how acidic or alkaline a solution. What Is An Acid Igcse.
From www.scribd.com
IGCSE Acid Base Concept PDF Acid Hydroxide What Is An Acid Igcse Study with quizlet and memorise flashcards. A neutralisation reaction occurs when an acid reacts with an alkali. The ph scale is a numerical scale which is used to show how acidic or alkaline a solution is. Define acids as proton donors and bases as proton acceptors. An acid is any substance that in water solution tastes sour, changes blue litmus. What Is An Acid Igcse.
From www.slideserve.com
PPT Naming Acids PowerPoint Presentation, free download ID3515861 What Is An Acid Igcse Examples of commonly known acids include. Study with quizlet and memorise flashcards. Define a strong acid as an acid that is completely dissociated in aqueous solution and. An acid is any substance that in water solution tastes sour, changes blue litmus paper to red, reacts with some metals to. A solution that contains the maximum amount of dissolved solute at. What Is An Acid Igcse.
From www.studypool.com
SOLUTION acid, base and salt igcse chemistry Studypool What Is An Acid Igcse The ph scale is a numerical scale which is used to show how acidic or alkaline a solution is. Define a strong acid as an acid that is completely dissociated in aqueous solution and. A series of free igcse chemistry lessons (cambridge igcse chemistry). A neutralisation reaction occurs when an acid reacts with an alkali. Study with quizlet and memorise. What Is An Acid Igcse.
From www.chegg.com
Solved 1. What is an acid and base according to a. What Is An Acid Igcse A solution that contains the maximum amount of dissolved solute at a specified temperature. A series of free igcse chemistry lessons (cambridge igcse chemistry). An acid is a compound which when dissolved in water produces hydrogen ions (h^+^) and are described as proton donors. Acids are substances that can donate a proton (h+ ion) when dissolved in water. Study with. What Is An Acid Igcse.
From www.studypool.com
SOLUTION acid, base and salt igcse chemistry Studypool What Is An Acid Igcse Acids are substances that can donate a proton (h+ ion) when dissolved in water. The ph scale is a numerical scale which is used to show how acidic or alkaline a solution is. An acid is a compound which when dissolved in water produces hydrogen ions (h^+^) and are described as proton donors. Study with quizlet and memorise flashcards. A. What Is An Acid Igcse.
From www.onlinemathlearning.com
Organic Chemistry IGCSE Chemistry (solutions, examples, worksheets What Is An Acid Igcse Acids are substances that can donate a proton (h+ ion) when dissolved in water. Examples of commonly known acids include. A neutralisation reaction occurs when an acid reacts with an alkali. A series of free igcse chemistry lessons (cambridge igcse chemistry). Define a strong acid as an acid that is completely dissociated in aqueous solution and. An acid is a. What Is An Acid Igcse.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry What Is An Acid Igcse A neutralisation reaction occurs when an acid reacts with an alkali. A series of free igcse chemistry lessons (cambridge igcse chemistry). An acid is any substance that in water solution tastes sour, changes blue litmus paper to red, reacts with some metals to. Acids are substances that can donate a proton (h+ ion) when dissolved in water. Study with quizlet. What Is An Acid Igcse.
From www.youtube.com
IGCSE Chemistry Rates of Reaction YouTube What Is An Acid Igcse A neutralisation reaction occurs when an acid reacts with an alkali. An acid is any substance that in water solution tastes sour, changes blue litmus paper to red, reacts with some metals to. Study with quizlet and memorise flashcards. A series of free igcse chemistry lessons (cambridge igcse chemistry). Acids are substances that can donate a proton (h+ ion) when. What Is An Acid Igcse.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:3.2.4 Practical Effect of What Is An Acid Igcse The ph scale is a numerical scale which is used to show how acidic or alkaline a solution is. Study with quizlet and memorise flashcards. A neutralisation reaction occurs when an acid reacts with an alkali. A series of free igcse chemistry lessons (cambridge igcse chemistry). Define a strong acid as an acid that is completely dissociated in aqueous solution. What Is An Acid Igcse.
From www.worksheetsplanet.com
What is an Acid Definition of Acid What Is An Acid Igcse An acid is a compound which when dissolved in water produces hydrogen ions (h^+^) and are described as proton donors. Study with quizlet and memorise flashcards. An acid is any substance that in water solution tastes sour, changes blue litmus paper to red, reacts with some metals to. The ph scale is a numerical scale which is used to show. What Is An Acid Igcse.
From www.youtube.com
IGCSE Chemistry Definition of Acid and Base YouTube What Is An Acid Igcse A neutralisation reaction occurs when an acid reacts with an alkali. Define acids as proton donors and bases as proton acceptors. An acid is any substance that in water solution tastes sour, changes blue litmus paper to red, reacts with some metals to. An acid is a compound which when dissolved in water produces hydrogen ions (h^+^) and are described. What Is An Acid Igcse.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 2.36 Understand that an Acid is a Proton Donor What Is An Acid Igcse An acid is a compound which when dissolved in water produces hydrogen ions (h^+^) and are described as proton donors. The ph scale is a numerical scale which is used to show how acidic or alkaline a solution is. Study with quizlet and memorise flashcards. A solution that contains the maximum amount of dissolved solute at a specified temperature. A. What Is An Acid Igcse.
From www.youtube.com
IGCSE in Seconds What is liquid (acid test) ratio? Shermann Foo What Is An Acid Igcse A neutralisation reaction occurs when an acid reacts with an alkali. An acid is a compound which when dissolved in water produces hydrogen ions (h^+^) and are described as proton donors. Acids are substances that can donate a proton (h+ ion) when dissolved in water. Examples of commonly known acids include. Define acids as proton donors and bases as proton. What Is An Acid Igcse.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:2.4.1 Metals Reacting with What Is An Acid Igcse Examples of commonly known acids include. A solution that contains the maximum amount of dissolved solute at a specified temperature. Define a strong acid as an acid that is completely dissociated in aqueous solution and. Study with quizlet and memorise flashcards. Define acids as proton donors and bases as proton acceptors. The ph scale is a numerical scale which is. What Is An Acid Igcse.
From igcsechemistryrevision.weebly.com
iGCSE CHEMISTRY REVISION HELP The Periodic Table What Is An Acid Igcse An acid is any substance that in water solution tastes sour, changes blue litmus paper to red, reacts with some metals to. Define acids as proton donors and bases as proton acceptors. The ph scale is a numerical scale which is used to show how acidic or alkaline a solution is. Define a strong acid as an acid that is. What Is An Acid Igcse.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations (GCSE) — the science hive What Is An Acid Igcse Examples of commonly known acids include. An acid is a compound which when dissolved in water produces hydrogen ions (h^+^) and are described as proton donors. Define acids as proton donors and bases as proton acceptors. An acid is any substance that in water solution tastes sour, changes blue litmus paper to red, reacts with some metals to. A series. What Is An Acid Igcse.
From www.reddit.com
From what I know...acid +base=salt +water..but the option confused me What Is An Acid Igcse A neutralisation reaction occurs when an acid reacts with an alkali. An acid is a compound which when dissolved in water produces hydrogen ions (h^+^) and are described as proton donors. A solution that contains the maximum amount of dissolved solute at a specified temperature. A series of free igcse chemistry lessons (cambridge igcse chemistry). Define a strong acid as. What Is An Acid Igcse.
From www.slideserve.com
PPT iGCSE chemistry Section 2 lesson 3 PowerPoint Presentation, free What Is An Acid Igcse A solution that contains the maximum amount of dissolved solute at a specified temperature. Acids are substances that can donate a proton (h+ ion) when dissolved in water. A series of free igcse chemistry lessons (cambridge igcse chemistry). An acid is any substance that in water solution tastes sour, changes blue litmus paper to red, reacts with some metals to.. What Is An Acid Igcse.
From www.studypool.com
SOLUTION Igcse chemistry 0620 sulphuric acid Studypool What Is An Acid Igcse The ph scale is a numerical scale which is used to show how acidic or alkaline a solution is. An acid is any substance that in water solution tastes sour, changes blue litmus paper to red, reacts with some metals to. A neutralisation reaction occurs when an acid reacts with an alkali. Acids are substances that can donate a proton. What Is An Acid Igcse.
From www.onlinemathlearning.com
Acid, Bases, Salts IGCSE Chemistry (solutions, examples, worksheets What Is An Acid Igcse A neutralisation reaction occurs when an acid reacts with an alkali. Examples of commonly known acids include. Study with quizlet and memorise flashcards. The ph scale is a numerical scale which is used to show how acidic or alkaline a solution is. An acid is a compound which when dissolved in water produces hydrogen ions (h^+^) and are described as. What Is An Acid Igcse.
From www.onlinenotesnepal.com
Acid, Base, and Salt in Class 10 Science Online Notes Nepal What Is An Acid Igcse Define a strong acid as an acid that is completely dissociated in aqueous solution and. Study with quizlet and memorise flashcards. A solution that contains the maximum amount of dissolved solute at a specified temperature. The ph scale is a numerical scale which is used to show how acidic or alkaline a solution is. Acids are substances that can donate. What Is An Acid Igcse.
From www.scribd.com
Acid Bases and Salts Igcse Chemistry 0620 PDF Acid Hydroxide What Is An Acid Igcse An acid is a compound which when dissolved in water produces hydrogen ions (h^+^) and are described as proton donors. A neutralisation reaction occurs when an acid reacts with an alkali. Define acids as proton donors and bases as proton acceptors. Acids are substances that can donate a proton (h+ ion) when dissolved in water. An acid is any substance. What Is An Acid Igcse.
From www.onlinemathlearning.com
Acid, Bases, Salts IGCSE Chemistry (solutions, examples, worksheets What Is An Acid Igcse A neutralisation reaction occurs when an acid reacts with an alkali. Acids are substances that can donate a proton (h+ ion) when dissolved in water. A solution that contains the maximum amount of dissolved solute at a specified temperature. Define a strong acid as an acid that is completely dissociated in aqueous solution and. An acid is any substance that. What Is An Acid Igcse.
From www.vrogue.co
Sodium Metal Reacts With Hydrochloric Acid vrogue.co What Is An Acid Igcse A solution that contains the maximum amount of dissolved solute at a specified temperature. Define a strong acid as an acid that is completely dissociated in aqueous solution and. Acids are substances that can donate a proton (h+ ion) when dissolved in water. An acid is a compound which when dissolved in water produces hydrogen ions (h^+^) and are described. What Is An Acid Igcse.
From igcse-chemistry-edexcel.blogspot.com
IGCSE Chemistry 4.9 describe experiments to carry out acidalkali What Is An Acid Igcse The ph scale is a numerical scale which is used to show how acidic or alkaline a solution is. A solution that contains the maximum amount of dissolved solute at a specified temperature. Define a strong acid as an acid that is completely dissociated in aqueous solution and. An acid is a compound which when dissolved in water produces hydrogen. What Is An Acid Igcse.
From www.youtube.com
iGCSE / GCSE Chemistry Carboxylic acids (14.15) YouTube What Is An Acid Igcse An acid is a compound which when dissolved in water produces hydrogen ions (h^+^) and are described as proton donors. A neutralisation reaction occurs when an acid reacts with an alkali. Study with quizlet and memorise flashcards. Define acids as proton donors and bases as proton acceptors. An acid is any substance that in water solution tastes sour, changes blue. What Is An Acid Igcse.
From www.savemyexams.com
AcidBase Titrations CIE IGCSE Chemistry Revision Notes 2023 What Is An Acid Igcse An acid is any substance that in water solution tastes sour, changes blue litmus paper to red, reacts with some metals to. Study with quizlet and memorise flashcards. A solution that contains the maximum amount of dissolved solute at a specified temperature. Define a strong acid as an acid that is completely dissociated in aqueous solution and. Examples of commonly. What Is An Acid Igcse.
From www.worksheetsplanet.com
Acid and Bases Differences What Is An Acid Igcse The ph scale is a numerical scale which is used to show how acidic or alkaline a solution is. Examples of commonly known acids include. An acid is any substance that in water solution tastes sour, changes blue litmus paper to red, reacts with some metals to. A neutralisation reaction occurs when an acid reacts with an alkali. A series. What Is An Acid Igcse.
From dokumen.tips
(PDF) Acids, Bases and Salts IGCSE STUDY BANKigcsestudybank.weebly What Is An Acid Igcse Study with quizlet and memorise flashcards. Acids are substances that can donate a proton (h+ ion) when dissolved in water. A series of free igcse chemistry lessons (cambridge igcse chemistry). Define a strong acid as an acid that is completely dissociated in aqueous solution and. An acid is a compound which when dissolved in water produces hydrogen ions (h^+^) and. What Is An Acid Igcse.