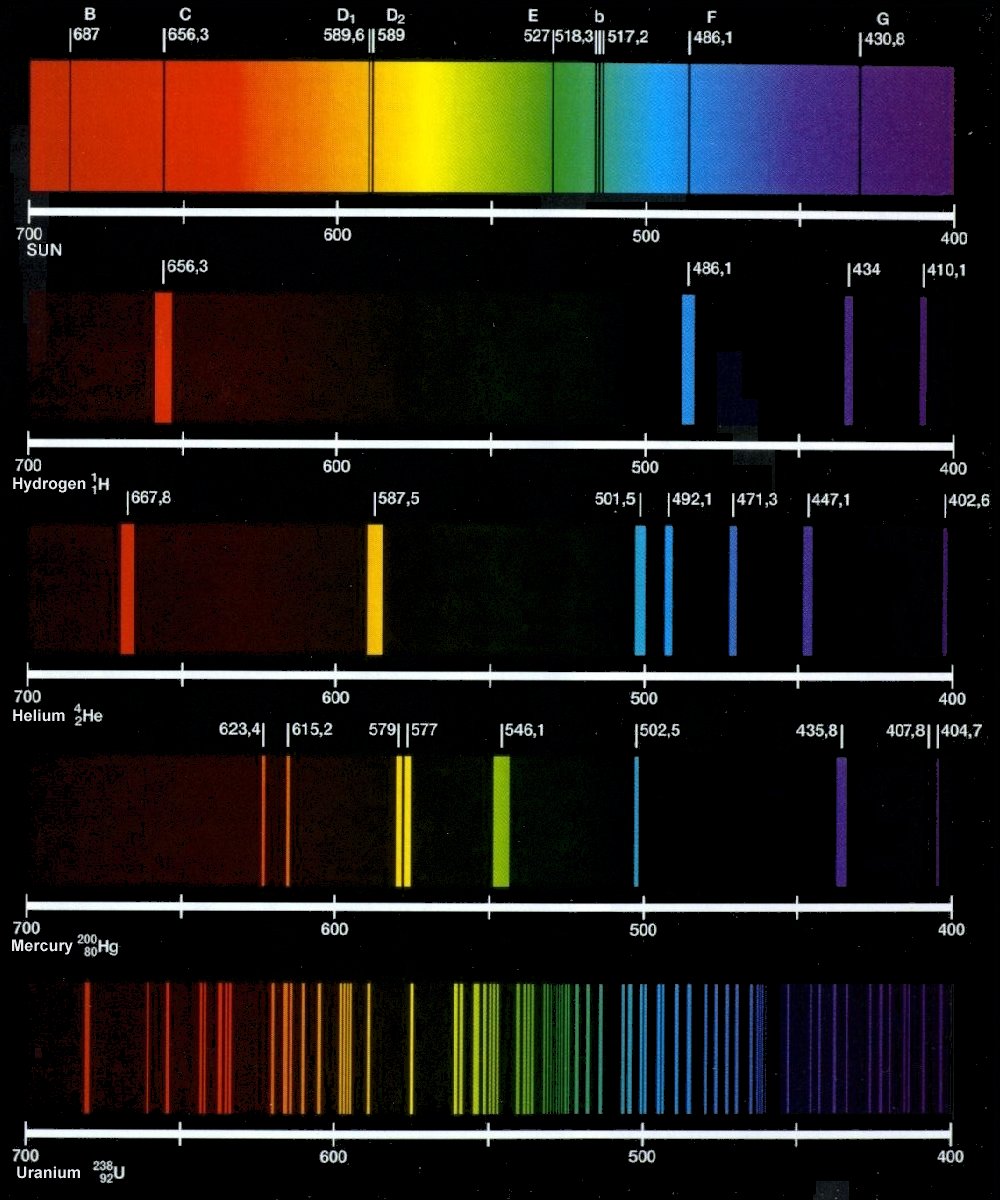
Spectroscopy Emission And Absorption . Emission spectrum & absorption spectrum. Starlight can also heat up a cloud. The main difference between emission and absorption spectra is that an emission spectrum has different coloured lines in the. Simplified illustration of absorption and emission spectra. Absorption is the process that consumes a photon and puts the atom or molecule in an excited state. Every element has a unique set of absorption and emission lines, or spectral signature. An absorption spectrum is like a photographic negative of an emission spectrum. Emission is the process that creates a photon and. For observing the absorption spectrum, electromagnetic radiations are. The absorption and emission spectra of each. An absorption spectrum has dark lines or gaps in the spectrum corresponding to wavelengths that are absorbed by the gas. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. The shapes of these emission spectra fall into two broad types: Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation.
from poozacreations.blogspot.com
Emission spectrum & absorption spectrum. The main difference between emission and absorption spectra is that an emission spectrum has different coloured lines in the. The absorption and emission spectra of each. Simplified illustration of absorption and emission spectra. The shapes of these emission spectra fall into two broad types: For observing the absorption spectrum, electromagnetic radiations are. An absorption spectrum is like a photographic negative of an emission spectrum. An absorption spectrum has dark lines or gaps in the spectrum corresponding to wavelengths that are absorbed by the gas. Emission is the process that creates a photon and. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation.
Types of emission and absorption spectra Pooza Creations
Spectroscopy Emission And Absorption Starlight can also heat up a cloud. Simplified illustration of absorption and emission spectra. Emission is the process that creates a photon and. For observing the absorption spectrum, electromagnetic radiations are. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. The absorption and emission spectra of each. The main difference between emission and absorption spectra is that an emission spectrum has different coloured lines in the. Starlight can also heat up a cloud. The shapes of these emission spectra fall into two broad types: Absorption is the process that consumes a photon and puts the atom or molecule in an excited state. Emission spectrum & absorption spectrum. Every element has a unique set of absorption and emission lines, or spectral signature. An absorption spectrum is like a photographic negative of an emission spectrum. An absorption spectrum has dark lines or gaps in the spectrum corresponding to wavelengths that are absorbed by the gas.
From www.slideserve.com
PPT Lecture 12 Introduction to Atomic Spectroscopy PowerPoint Spectroscopy Emission And Absorption The shapes of these emission spectra fall into two broad types: An absorption spectrum is like a photographic negative of an emission spectrum. Every element has a unique set of absorption and emission lines, or spectral signature. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on. Spectroscopy Emission And Absorption.
From vqg1811-700.com
What are Absorption, Excitation and Emission Spectra? Tech Blog Spectroscopy Emission And Absorption The absorption and emission spectra of each. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. The shapes of these emission spectra fall into two broad types: Every element has a unique set of absorption and emission lines, or spectral signature.. Spectroscopy Emission And Absorption.
From www.youtube.com
Absorption spectrum and Emission spectrum video YouTube Spectroscopy Emission And Absorption The main difference between emission and absorption spectra is that an emission spectrum has different coloured lines in the. The shapes of these emission spectra fall into two broad types: Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. Emission is. Spectroscopy Emission And Absorption.
From users.highland.edu
Atomic Spectra and Models of the Atom Spectroscopy Emission And Absorption An absorption spectrum has dark lines or gaps in the spectrum corresponding to wavelengths that are absorbed by the gas. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on. Spectroscopy Emission And Absorption.
From webbtelescope.org
Types of Spectra Continuous, Emission, and Absorption b Spectroscopy Emission And Absorption Simplified illustration of absorption and emission spectra. An absorption spectrum is like a photographic negative of an emission spectrum. The shapes of these emission spectra fall into two broad types: For observing the absorption spectrum, electromagnetic radiations are. An absorption spectrum has dark lines or gaps in the spectrum corresponding to wavelengths that are absorbed by the gas. Emission spectrum. Spectroscopy Emission And Absorption.
From www.pinterest.com
Emission vs Absorption Spectrum Thursday, March 29, 2018 Earth and Spectroscopy Emission And Absorption Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. An absorption spectrum has dark lines or gaps in the spectrum corresponding to wavelengths that are absorbed by the gas. Absorption is the process that consumes a photon and puts the atom. Spectroscopy Emission And Absorption.
From www.slideserve.com
PPT Lecture 12 Introduction to Atomic Spectroscopy PowerPoint Spectroscopy Emission And Absorption An absorption spectrum is like a photographic negative of an emission spectrum. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. An absorption spectrum has dark lines or gaps in the spectrum corresponding to wavelengths that are absorbed by the gas.. Spectroscopy Emission And Absorption.
From www.youtube.com
Difference Between Absorption and Emission Spectra YouTube Spectroscopy Emission And Absorption An absorption spectrum has dark lines or gaps in the spectrum corresponding to wavelengths that are absorbed by the gas. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Every element has a unique set of absorption and emission lines, or spectral signature. Emission spectrum & absorption spectrum. Simplified illustration of absorption. Spectroscopy Emission And Absorption.
From www.researchgate.net
The absorption and emission spectra of Rhodamine 6G, a fluorescent Spectroscopy Emission And Absorption The absorption and emission spectra of each. The shapes of these emission spectra fall into two broad types: Starlight can also heat up a cloud. Emission spectrum & absorption spectrum. Absorption is the process that consumes a photon and puts the atom or molecule in an excited state. An absorption spectrum is like a photographic negative of an emission spectrum.. Spectroscopy Emission And Absorption.
From byjus.com
Emission Spectrum Atomic Spectra Comparison Chemistry Byju's Spectroscopy Emission And Absorption An absorption spectrum is like a photographic negative of an emission spectrum. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. The main difference. Spectroscopy Emission And Absorption.
From www.toppr.com
Define emission spectron and absorption spectrum. Spectroscopy Emission And Absorption An absorption spectrum is like a photographic negative of an emission spectrum. Absorption is the process that consumes a photon and puts the atom or molecule in an excited state. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Every element has a unique set of absorption and emission lines, or spectral. Spectroscopy Emission And Absorption.
From www.astronoo.com
Spectroscopy — Astronoo Spectroscopy Emission And Absorption Emission spectrum & absorption spectrum. For observing the absorption spectrum, electromagnetic radiations are. Simplified illustration of absorption and emission spectra. Every element has a unique set of absorption and emission lines, or spectral signature. An absorption spectrum has dark lines or gaps in the spectrum corresponding to wavelengths that are absorbed by the gas. Starlight can also heat up a. Spectroscopy Emission And Absorption.
From www.slideshare.net
Atomic Spectroscopy Basic Principles and Instruments Spectroscopy Emission And Absorption Starlight can also heat up a cloud. For observing the absorption spectrum, electromagnetic radiations are. Every element has a unique set of absorption and emission lines, or spectral signature. An absorption spectrum has dark lines or gaps in the spectrum corresponding to wavelengths that are absorbed by the gas. The main difference between emission and absorption spectra is that an. Spectroscopy Emission And Absorption.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Spectroscopy Emission And Absorption Emission is the process that creates a photon and. An absorption spectrum has dark lines or gaps in the spectrum corresponding to wavelengths that are absorbed by the gas. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. The absorption and. Spectroscopy Emission And Absorption.
From webbtelescope.org
Absorption and Emission Spectra of Various Elements b Spectroscopy Emission And Absorption The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Absorption is the process that consumes a photon and puts the atom or molecule in an excited state. Every element has a unique set of absorption and emission lines, or spectral signature. An absorption spectrum is like a photographic negative of an emission. Spectroscopy Emission And Absorption.
From es.slideshare.net
Emission spectroscopy Spectroscopy Emission And Absorption Every element has a unique set of absorption and emission lines, or spectral signature. The shapes of these emission spectra fall into two broad types: Simplified illustration of absorption and emission spectra. The main difference between emission and absorption spectra is that an emission spectrum has different coloured lines in the. Emission is the process that creates a photon and.. Spectroscopy Emission And Absorption.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Spectroscopy Emission And Absorption Emission spectrum & absorption spectrum. The shapes of these emission spectra fall into two broad types: Starlight can also heat up a cloud. An absorption spectrum has dark lines or gaps in the spectrum corresponding to wavelengths that are absorbed by the gas. Every element has a unique set of absorption and emission lines, or spectral signature. Emission is the. Spectroscopy Emission And Absorption.
From www.slideserve.com
PPT CHEMISTRY XL14A NATURE OF LIGHT AND THE ATOM PowerPoint Spectroscopy Emission And Absorption The main difference between emission and absorption spectra is that an emission spectrum has different coloured lines in the. Every element has a unique set of absorption and emission lines, or spectral signature. Absorption is the process that consumes a photon and puts the atom or molecule in an excited state. Spectroscopy, study of the absorption and emission of light. Spectroscopy Emission And Absorption.
From www.slideshare.net
Chapter 3 Arrangement of Electrons in the Atom Spectroscopy Emission And Absorption Absorption is the process that consumes a photon and puts the atom or molecule in an excited state. The main difference between emission and absorption spectra is that an emission spectrum has different coloured lines in the. An absorption spectrum has dark lines or gaps in the spectrum corresponding to wavelengths that are absorbed by the gas. Emission is the. Spectroscopy Emission And Absorption.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Spectroscopy Emission And Absorption Emission is the process that creates a photon and. Simplified illustration of absorption and emission spectra. The main difference between emission and absorption spectra is that an emission spectrum has different coloured lines in the. The absorption and emission spectra of each. Every element has a unique set of absorption and emission lines, or spectral signature. The shapes of these. Spectroscopy Emission And Absorption.
From protonstalk.com
Difference Between Emission and Absorption Spectra ProtonsTalk Spectroscopy Emission And Absorption Emission spectrum & absorption spectrum. Absorption is the process that consumes a photon and puts the atom or molecule in an excited state. The shapes of these emission spectra fall into two broad types: The main difference between emission and absorption spectra is that an emission spectrum has different coloured lines in the. For observing the absorption spectrum, electromagnetic radiations. Spectroscopy Emission And Absorption.
From www.youtube.com
Types of Spectroscopy Atomic and Molecular Absorption and Emission Spectroscopy Emission And Absorption Starlight can also heat up a cloud. An absorption spectrum has dark lines or gaps in the spectrum corresponding to wavelengths that are absorbed by the gas. The shapes of these emission spectra fall into two broad types: Emission spectrum & absorption spectrum. The result is an emission spectrum that shows the intensity of emission as a function of wavelength.. Spectroscopy Emission And Absorption.
From www.nagwa.com
Lesson Video Emission and Absorption Spectra Nagwa Spectroscopy Emission And Absorption The shapes of these emission spectra fall into two broad types: Emission is the process that creates a photon and. An absorption spectrum is like a photographic negative of an emission spectrum. Emission spectrum & absorption spectrum. The absorption and emission spectra of each. Starlight can also heat up a cloud. Spectroscopy, study of the absorption and emission of light. Spectroscopy Emission And Absorption.
From pixels.com
Helium Emission And Absorption Spectra Photograph by Carlos Clarivan Spectroscopy Emission And Absorption The shapes of these emission spectra fall into two broad types: For observing the absorption spectrum, electromagnetic radiations are. Starlight can also heat up a cloud. Emission is the process that creates a photon and. The main difference between emission and absorption spectra is that an emission spectrum has different coloured lines in the. The absorption and emission spectra of. Spectroscopy Emission And Absorption.
From www.priyamstudycentre.com
Spectroscopy Definition, Types, Applications Spectroscopy Emission And Absorption Emission is the process that creates a photon and. Every element has a unique set of absorption and emission lines, or spectral signature. The main difference between emission and absorption spectra is that an emission spectrum has different coloured lines in the. The shapes of these emission spectra fall into two broad types: The result is an emission spectrum that. Spectroscopy Emission And Absorption.
From studylib.net
Absorption, Emission and Fluorescence Spectroscopies Spectroscopy Emission And Absorption Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. Starlight can also heat up a cloud. For observing the absorption spectrum, electromagnetic radiations are. Absorption is the process that consumes a photon and puts the atom or molecule in an excited. Spectroscopy Emission And Absorption.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum Spectroscopy Emission And Absorption The absorption and emission spectra of each. An absorption spectrum has dark lines or gaps in the spectrum corresponding to wavelengths that are absorbed by the gas. For observing the absorption spectrum, electromagnetic radiations are. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength. Spectroscopy Emission And Absorption.
From pediaa.com
Difference Between Absorption and Emission Spectroscopy Emission And Absorption The shapes of these emission spectra fall into two broad types: An absorption spectrum is like a photographic negative of an emission spectrum. The main difference between emission and absorption spectra is that an emission spectrum has different coloured lines in the. The absorption and emission spectra of each. The result is an emission spectrum that shows the intensity of. Spectroscopy Emission And Absorption.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Spectroscopy Emission And Absorption Starlight can also heat up a cloud. Absorption is the process that consumes a photon and puts the atom or molecule in an excited state. Simplified illustration of absorption and emission spectra. An absorption spectrum is like a photographic negative of an emission spectrum. Emission spectrum & absorption spectrum. For observing the absorption spectrum, electromagnetic radiations are. Spectroscopy, study of. Spectroscopy Emission And Absorption.
From www.researchgate.net
Absorption and emission spectrum for a 10− 5 M concentration of quinine Spectroscopy Emission And Absorption The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Every element has a unique set of absorption and emission lines, or spectral signature. The main difference between emission and absorption spectra is that an emission spectrum has different coloured lines in the. An absorption spectrum is like a photographic negative of an. Spectroscopy Emission And Absorption.
From www.priyamstudycentre.com
Atomic Emission Spectroscopy Inductively Coupled Plasma Spectroscopy Emission And Absorption The absorption and emission spectra of each. Simplified illustration of absorption and emission spectra. Absorption is the process that consumes a photon and puts the atom or molecule in an excited state. For observing the absorption spectrum, electromagnetic radiations are. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of. Spectroscopy Emission And Absorption.
From whitsonmysecutage.blogspot.com
Continuous Spectrum Vs Emission Spectrum Vs Absorption Spectrum Spectroscopy Emission And Absorption The shapes of these emission spectra fall into two broad types: Simplified illustration of absorption and emission spectra. The main difference between emission and absorption spectra is that an emission spectrum has different coloured lines in the. For observing the absorption spectrum, electromagnetic radiations are. Emission spectrum & absorption spectrum. The result is an emission spectrum that shows the intensity. Spectroscopy Emission And Absorption.
From www.pinterest.com
Emission Spectrum Vs. Absorption Spectrum Astronomy lessons Spectroscopy Emission And Absorption Absorption is the process that consumes a photon and puts the atom or molecule in an excited state. Simplified illustration of absorption and emission spectra. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. The shapes of these emission spectra fall into two broad types: Emission is the process that creates a. Spectroscopy Emission And Absorption.
From www.researchgate.net
Absorption and fluorescence (excitation and emission) spectra of (3) in Spectroscopy Emission And Absorption The result is an emission spectrum that shows the intensity of emission as a function of wavelength. An absorption spectrum has dark lines or gaps in the spectrum corresponding to wavelengths that are absorbed by the gas. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on. Spectroscopy Emission And Absorption.
From crunchchemistry.co.uk
Atomic absorption and emission spectroscopy Crunch Chemistry Spectroscopy Emission And Absorption Starlight can also heat up a cloud. The main difference between emission and absorption spectra is that an emission spectrum has different coloured lines in the. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. Absorption is the process that consumes. Spectroscopy Emission And Absorption.