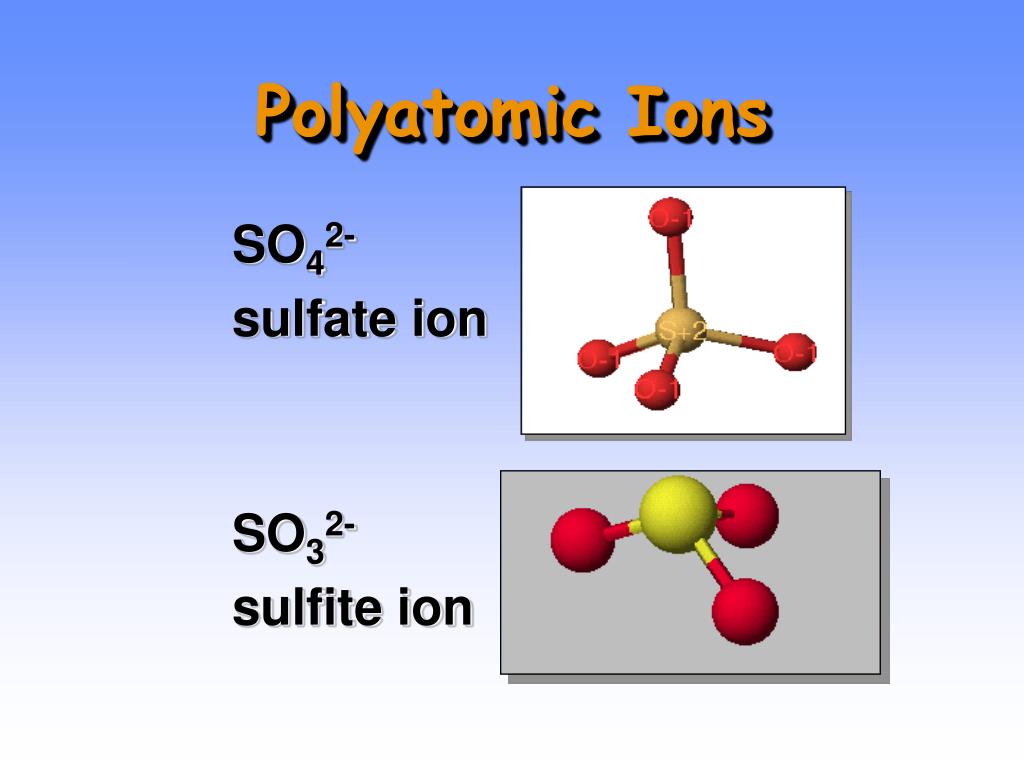
Aluminum Sulfate Polyatomic Ion . Table \(\pageindex{1}\) lists the ion. rules for naming ionic compounds containing polyatomic ions. Aluminium sulfate is made by adding aluminium hydroxide i.e., al(oh)3, to sulfuric acid i.e., h2so4. How to name ionic compounds containing common polyatomic ions. Polyatomic ions are ions which consist of more than one atom. writing formulas for ionic compounds containing polyatomic ions. because these ions contain more than one atom, they are called polyatomic ions. 2al(oh)3 + 3h2so4 → al2(so4)3 + 6h2o. Polyatomic ions have defined formulas, names, and charges that cannot be modified in any way. For example, no 3 − is the. Names, formula, and charges of polyatomic ions. Aluminum is a group 3a metal which forms a #3+# cation. Writing formulas for ionic compounds containing polyatomic ions involves the same steps as for a binary ionic compound. The cation is written first. Or it is done by heating aluminium metal in a sulfuric acid.
from www.slideserve.com
2al(oh)3 + 3h2so4 → al2(so4)3 + 6h2o. Aluminum is a group 3a metal which forms a #3+# cation. Or it is done by heating aluminium metal in a sulfuric acid. The cation is written first. Aluminium sulfate is made by adding aluminium hydroxide i.e., al(oh)3, to sulfuric acid i.e., h2so4. aluminium sulphate is an ionic. A trick to balancing the charge is to use the number of the charge of one ion as the subscript. Names, formula, and charges of polyatomic ions. How to name ionic compounds containing common polyatomic ions. rules for naming ionic compounds containing polyatomic ions.
PPT Compounds & Molecules PowerPoint Presentation, free download ID
Aluminum Sulfate Polyatomic Ion The cation is written first. Polyatomic ions are ions which consist of more than one atom. aluminium sulphate is an ionic. rules for naming ionic compounds containing polyatomic ions. Names, formula, and charges of polyatomic ions. Table \(\pageindex{1}\) lists the ion. How to name ionic compounds containing common polyatomic ions. For example, no 3 − is the. 2al(oh)3 + 3h2so4 → al2(so4)3 + 6h2o. Aluminium sulfate is made by adding aluminium hydroxide i.e., al(oh)3, to sulfuric acid i.e., h2so4. The cation is written first. Polyatomic ions have characteristic formulas, names, and charges that should be memorized. A trick to balancing the charge is to use the number of the charge of one ion as the subscript. Writing formulas for ionic compounds containing polyatomic ions involves the same steps as for a binary ionic compound. Or it is done by heating aluminium metal in a sulfuric acid. writing formulas for ionic compounds containing polyatomic ions.
From reviewhomedecor.co
Periodic Table With Charges And Polyatomic Ions Review Home Decor Aluminum Sulfate Polyatomic Ion Or it is done by heating aluminium metal in a sulfuric acid. Aluminum is a group 3a metal which forms a #3+# cation. In this article, we will discuss polyatomic ions. For example, no 3 − is the. Polyatomic ions have defined formulas, names, and charges that cannot be modified in any way. Aluminium sulfate is made by adding aluminium. Aluminum Sulfate Polyatomic Ion.
From www.numerade.com
SOLVED NAMES, FORMULAS, AND CHARGES OF POLYATOMIC IONS In the same way Aluminum Sulfate Polyatomic Ion For example, no 3 − is the. Polyatomic ions have characteristic formulas, names, and charges that should be memorized. 2al(oh)3 + 3h2so4 → al2(so4)3 + 6h2o. Aluminium sulfate is made by adding aluminium hydroxide i.e., al(oh)3, to sulfuric acid i.e., h2so4. Writing formulas for ionic compounds containing polyatomic ions involves the same steps as for a binary ionic compound. A. Aluminum Sulfate Polyatomic Ion.
From www.jinleichem.com
Aluminum Sulfate Jinlei Chemical Aluminum Sulfate Polyatomic Ion Aluminium sulfate is made by adding aluminium hydroxide i.e., al(oh)3, to sulfuric acid i.e., h2so4. Polyatomic ions are ions which consist of more than one atom. 2al(oh)3 + 3h2so4 → al2(so4)3 + 6h2o. aluminium sulphate is an ionic. For example, no 3 − is the. Polyatomic ions have defined formulas, names, and charges that cannot be modified in any. Aluminum Sulfate Polyatomic Ion.
From www.numerade.com
SOLVEDWrite the formula for each of the following compounds that Aluminum Sulfate Polyatomic Ion Polyatomic ions have defined formulas, names, and charges that cannot be modified in any way. Writing formulas for ionic compounds containing polyatomic ions involves the same steps as for a binary ionic compound. Aluminium sulfate is made by adding aluminium hydroxide i.e., al(oh)3, to sulfuric acid i.e., h2so4. writing formulas for ionic compounds containing polyatomic ions. For example, no. Aluminum Sulfate Polyatomic Ion.
From aluminumsulfatepitsukata.blogspot.com
Aluminum Sulfate Aluminum Sulfate Nomenclature Aluminum Sulfate Polyatomic Ion because these ions contain more than one atom, they are called polyatomic ions. The cation is written first. For example, no 3 − is the. Polyatomic ions have characteristic formulas, names, and charges that should be memorized. rules for naming ionic compounds containing polyatomic ions. How to name ionic compounds containing common polyatomic ions. In this article, we. Aluminum Sulfate Polyatomic Ion.
From www.numerade.com
SOLVED TERNARY IONIC COMPOUNDS OF METALS POLYATOMIC IONS (TABLE 3 Aluminum Sulfate Polyatomic Ion rules for naming ionic compounds containing polyatomic ions. because these ions contain more than one atom, they are called polyatomic ions. A trick to balancing the charge is to use the number of the charge of one ion as the subscript. writing formulas for ionic compounds containing polyatomic ions. Table \(\pageindex{1}\) lists the ion. aluminium sulphate. Aluminum Sulfate Polyatomic Ion.
From www.chegg.com
Solved 1. Sulfate (SO42) is a common polyatomic ion. Given Aluminum Sulfate Polyatomic Ion writing formulas for ionic compounds containing polyatomic ions. aluminium sulphate is an ionic. How to name ionic compounds containing common polyatomic ions. rules for naming ionic compounds containing polyatomic ions. because these ions contain more than one atom, they are called polyatomic ions. Aluminum is a group 3a metal which forms a #3+# cation. Polyatomic ions. Aluminum Sulfate Polyatomic Ion.
From ar.inspiredpencil.com
Polyatomic Ions Chart Aluminum Sulfate Polyatomic Ion aluminium sulphate is an ionic. Names, formula, and charges of polyatomic ions. Polyatomic ions have characteristic formulas, names, and charges that should be memorized. In this article, we will discuss polyatomic ions. Aluminum is a group 3a metal which forms a #3+# cation. Table \(\pageindex{1}\) lists the ion. Writing formulas for ionic compounds containing polyatomic ions involves the same. Aluminum Sulfate Polyatomic Ion.
From www.findwordtemplates.com
Polyatomic Ion Charts Find Word Templates Aluminum Sulfate Polyatomic Ion because these ions contain more than one atom, they are called polyatomic ions. rules for naming ionic compounds containing polyatomic ions. aluminium sulphate is an ionic. How to name ionic compounds containing common polyatomic ions. In this article, we will discuss polyatomic ions. Polyatomic ions have characteristic formulas, names, and charges that should be memorized. Table \(\pageindex{1}\). Aluminum Sulfate Polyatomic Ion.
From www.slideserve.com
PPT Polyatomic Ions and Their Compounds PowerPoint Presentation, free Aluminum Sulfate Polyatomic Ion Table \(\pageindex{1}\) lists the ion. For example, no 3 − is the. because these ions contain more than one atom, they are called polyatomic ions. A trick to balancing the charge is to use the number of the charge of one ion as the subscript. How to name ionic compounds containing common polyatomic ions. Or it is done by. Aluminum Sulfate Polyatomic Ion.
From www.pinterest.com
polyatomicions A list of the names and formulas of some common Aluminum Sulfate Polyatomic Ion rules for naming ionic compounds containing polyatomic ions. In this article, we will discuss polyatomic ions. Writing formulas for ionic compounds containing polyatomic ions involves the same steps as for a binary ionic compound. writing formulas for ionic compounds containing polyatomic ions. Table \(\pageindex{1}\) lists the ion. Names, formula, and charges of polyatomic ions. Aluminium sulfate is made. Aluminum Sulfate Polyatomic Ion.
From www.slideserve.com
PPT Polyatomic Ions and Their Compounds PowerPoint Presentation, free Aluminum Sulfate Polyatomic Ion Polyatomic ions are ions which consist of more than one atom. aluminium sulphate is an ionic. rules for naming ionic compounds containing polyatomic ions. In this article, we will discuss polyatomic ions. Polyatomic ions have defined formulas, names, and charges that cannot be modified in any way. Writing formulas for ionic compounds containing polyatomic ions involves the same. Aluminum Sulfate Polyatomic Ion.
From www.pinterest.com
How Do You Teach Molar Mass? in 2021 Molar mass, Teaching, Science Aluminum Sulfate Polyatomic Ion For example, no 3 − is the. Table \(\pageindex{1}\) lists the ion. In this article, we will discuss polyatomic ions. Polyatomic ions are ions which consist of more than one atom. Writing formulas for ionic compounds containing polyatomic ions involves the same steps as for a binary ionic compound. Aluminium sulfate is made by adding aluminium hydroxide i.e., al(oh)3, to. Aluminum Sulfate Polyatomic Ion.
From learnwithdrscott.com
Polyatomic Ions List and Worksheet Easy Hard Science Aluminum Sulfate Polyatomic Ion 2al(oh)3 + 3h2so4 → al2(so4)3 + 6h2o. The cation is written first. Polyatomic ions are ions which consist of more than one atom. Polyatomic ions have defined formulas, names, and charges that cannot be modified in any way. For example, no 3 − is the. rules for naming ionic compounds containing polyatomic ions. Polyatomic ions have characteristic formulas, names,. Aluminum Sulfate Polyatomic Ion.
From www.youtube.com
Is Al2(SO4)3 (Aluminum sulfate) Ionic or Covalent? YouTube Aluminum Sulfate Polyatomic Ion Polyatomic ions have defined formulas, names, and charges that cannot be modified in any way. Or it is done by heating aluminium metal in a sulfuric acid. 2al(oh)3 + 3h2so4 → al2(so4)3 + 6h2o. Aluminium sulfate is made by adding aluminium hydroxide i.e., al(oh)3, to sulfuric acid i.e., h2so4. aluminium sulphate is an ionic. Table \(\pageindex{1}\) lists the ion.. Aluminum Sulfate Polyatomic Ion.
From www.slideserve.com
PPT Ionic Nomenclature PowerPoint Presentation ID2031508 Aluminum Sulfate Polyatomic Ion A trick to balancing the charge is to use the number of the charge of one ion as the subscript. For example, no 3 − is the. Writing formulas for ionic compounds containing polyatomic ions involves the same steps as for a binary ionic compound. Polyatomic ions have defined formulas, names, and charges that cannot be modified in any way.. Aluminum Sulfate Polyatomic Ion.
From www.numerade.com
SOLVED Aluminum sulfate, Al2(SO4)3, to form aluminum oxide Aluminum Sulfate Polyatomic Ion A trick to balancing the charge is to use the number of the charge of one ion as the subscript. Names, formula, and charges of polyatomic ions. aluminium sulphate is an ionic. Polyatomic ions have characteristic formulas, names, and charges that should be memorized. How to name ionic compounds containing common polyatomic ions. Table \(\pageindex{1}\) lists the ion. Aluminum. Aluminum Sulfate Polyatomic Ion.
From imgbin.com
Sulfate Polyatomic Ion Lewis Structure Chemical Bond PNG, Clipart Aluminum Sulfate Polyatomic Ion Polyatomic ions have characteristic formulas, names, and charges that should be memorized. The cation is written first. How to name ionic compounds containing common polyatomic ions. writing formulas for ionic compounds containing polyatomic ions. because these ions contain more than one atom, they are called polyatomic ions. rules for naming ionic compounds containing polyatomic ions. Writing formulas. Aluminum Sulfate Polyatomic Ion.
From brainly.in
Write the formulae of three polyatomic radicals. Brainly.in Aluminum Sulfate Polyatomic Ion Names, formula, and charges of polyatomic ions. Or it is done by heating aluminium metal in a sulfuric acid. 2al(oh)3 + 3h2so4 → al2(so4)3 + 6h2o. For example, no 3 − is the. Writing formulas for ionic compounds containing polyatomic ions involves the same steps as for a binary ionic compound. aluminium sulphate is an ionic. Polyatomic ions have. Aluminum Sulfate Polyatomic Ion.
From www.indiamart.com
Aluminum Sulphate at best price in Vapi by Chemtrron Corporation ID Aluminum Sulfate Polyatomic Ion rules for naming ionic compounds containing polyatomic ions. Aluminium sulfate is made by adding aluminium hydroxide i.e., al(oh)3, to sulfuric acid i.e., h2so4. Names, formula, and charges of polyatomic ions. Table \(\pageindex{1}\) lists the ion. Polyatomic ions are ions which consist of more than one atom. because these ions contain more than one atom, they are called polyatomic. Aluminum Sulfate Polyatomic Ion.
From www.slideserve.com
PPT AND PowerPoint Presentation, free download ID2959451 Aluminum Sulfate Polyatomic Ion Polyatomic ions are ions which consist of more than one atom. Or it is done by heating aluminium metal in a sulfuric acid. Aluminum is a group 3a metal which forms a #3+# cation. because these ions contain more than one atom, they are called polyatomic ions. Polyatomic ions have characteristic formulas, names, and charges that should be memorized.. Aluminum Sulfate Polyatomic Ion.
From www.inspiritvr.com
Polyatomic Ions Naming and Formulas Study Guide Inspirit Aluminum Sulfate Polyatomic Ion Polyatomic ions have defined formulas, names, and charges that cannot be modified in any way. Aluminum is a group 3a metal which forms a #3+# cation. writing formulas for ionic compounds containing polyatomic ions. because these ions contain more than one atom, they are called polyatomic ions. Aluminium sulfate is made by adding aluminium hydroxide i.e., al(oh)3, to. Aluminum Sulfate Polyatomic Ion.
From rahaoil.com
Aluminum Sulfate Aluminum Sulphate Manufacturer RAHA Oil Co. Aluminum Sulfate Polyatomic Ion Polyatomic ions are ions which consist of more than one atom. Table \(\pageindex{1}\) lists the ion. 2al(oh)3 + 3h2so4 → al2(so4)3 + 6h2o. In this article, we will discuss polyatomic ions. because these ions contain more than one atom, they are called polyatomic ions. A trick to balancing the charge is to use the number of the charge of. Aluminum Sulfate Polyatomic Ion.
From byjus.com
Write down the formula of sodium sulphate using the Criss cross method. Aluminum Sulfate Polyatomic Ion Aluminium sulfate is made by adding aluminium hydroxide i.e., al(oh)3, to sulfuric acid i.e., h2so4. Writing formulas for ionic compounds containing polyatomic ions involves the same steps as for a binary ionic compound. aluminium sulphate is an ionic. writing formulas for ionic compounds containing polyatomic ions. 2al(oh)3 + 3h2so4 → al2(so4)3 + 6h2o. Aluminum is a group 3a. Aluminum Sulfate Polyatomic Ion.
From fr.slideserve.com
PPT Common Polyatomic Ions PowerPoint Presentation, free download Aluminum Sulfate Polyatomic Ion Writing formulas for ionic compounds containing polyatomic ions involves the same steps as for a binary ionic compound. Aluminum is a group 3a metal which forms a #3+# cation. because these ions contain more than one atom, they are called polyatomic ions. Polyatomic ions are ions which consist of more than one atom. Table \(\pageindex{1}\) lists the ion. . Aluminum Sulfate Polyatomic Ion.
From www.vlr.eng.br
Sulfide, Sulfite, Sulfate Ions (Difference And Formulas) vlr.eng.br Aluminum Sulfate Polyatomic Ion Polyatomic ions have characteristic formulas, names, and charges that should be memorized. Or it is done by heating aluminium metal in a sulfuric acid. because these ions contain more than one atom, they are called polyatomic ions. A trick to balancing the charge is to use the number of the charge of one ion as the subscript. Table \(\pageindex{1}\). Aluminum Sulfate Polyatomic Ion.
From ftwhomeworkobu.web.fc2.com
Write a molecular equation for aluminum sulfate and sodium phosphate Aluminum Sulfate Polyatomic Ion aluminium sulphate is an ionic. because these ions contain more than one atom, they are called polyatomic ions. Writing formulas for ionic compounds containing polyatomic ions involves the same steps as for a binary ionic compound. The cation is written first. 2al(oh)3 + 3h2so4 → al2(so4)3 + 6h2o. How to name ionic compounds containing common polyatomic ions. . Aluminum Sulfate Polyatomic Ion.
From www.bartleby.com
Answered Part 2 Polyatomic ions Complete the… bartleby Aluminum Sulfate Polyatomic Ion Polyatomic ions have defined formulas, names, and charges that cannot be modified in any way. 2al(oh)3 + 3h2so4 → al2(so4)3 + 6h2o. Writing formulas for ionic compounds containing polyatomic ions involves the same steps as for a binary ionic compound. rules for naming ionic compounds containing polyatomic ions. The cation is written first. Aluminum is a group 3a metal. Aluminum Sulfate Polyatomic Ion.
From www.slideserve.com
PPT Writing Ionic Formulas PowerPoint Presentation, free download Aluminum Sulfate Polyatomic Ion Polyatomic ions are ions which consist of more than one atom. Names, formula, and charges of polyatomic ions. rules for naming ionic compounds containing polyatomic ions. Aluminum is a group 3a metal which forms a #3+# cation. because these ions contain more than one atom, they are called polyatomic ions. For example, no 3 − is the. Or. Aluminum Sulfate Polyatomic Ion.
From www.indiamart.com
Aluminum Sulphate, Aluminum Sulfate, Aluminum Sulphate, Aluminium Aluminum Sulfate Polyatomic Ion Polyatomic ions have characteristic formulas, names, and charges that should be memorized. Polyatomic ions have defined formulas, names, and charges that cannot be modified in any way. Aluminum is a group 3a metal which forms a #3+# cation. because these ions contain more than one atom, they are called polyatomic ions. Aluminium sulfate is made by adding aluminium hydroxide. Aluminum Sulfate Polyatomic Ion.
From www.slideserve.com
PPT Compounds & Molecules PowerPoint Presentation, free download ID Aluminum Sulfate Polyatomic Ion Polyatomic ions are ions which consist of more than one atom. For example, no 3 − is the. rules for naming ionic compounds containing polyatomic ions. aluminium sulphate is an ionic. Names, formula, and charges of polyatomic ions. Writing formulas for ionic compounds containing polyatomic ions involves the same steps as for a binary ionic compound. Table \(\pageindex{1}\). Aluminum Sulfate Polyatomic Ion.
From quizlet.com
polyatomic ions Diagram Quizlet Aluminum Sulfate Polyatomic Ion Polyatomic ions have characteristic formulas, names, and charges that should be memorized. Or it is done by heating aluminium metal in a sulfuric acid. The cation is written first. Aluminum is a group 3a metal which forms a #3+# cation. A trick to balancing the charge is to use the number of the charge of one ion as the subscript.. Aluminum Sulfate Polyatomic Ion.
From www.pinterest.at
Common Polyatomic ions Teaching chemistry, Chemistry lessons Aluminum Sulfate Polyatomic Ion 2al(oh)3 + 3h2so4 → al2(so4)3 + 6h2o. A trick to balancing the charge is to use the number of the charge of one ion as the subscript. Aluminum is a group 3a metal which forms a #3+# cation. Polyatomic ions are ions which consist of more than one atom. because these ions contain more than one atom, they are. Aluminum Sulfate Polyatomic Ion.
From www.inoxia.co.uk
Buy Aluminium Sulphate at Inoxia Ltd Aluminum Sulfate Polyatomic Ion rules for naming ionic compounds containing polyatomic ions. How to name ionic compounds containing common polyatomic ions. Aluminum is a group 3a metal which forms a #3+# cation. For example, no 3 − is the. Writing formulas for ionic compounds containing polyatomic ions involves the same steps as for a binary ionic compound. aluminium sulphate is an ionic.. Aluminum Sulfate Polyatomic Ion.
From www.teachoo.com
What are Polyatomic Ions? Give Examples Teachoo Concepts Aluminum Sulfate Polyatomic Ion Or it is done by heating aluminium metal in a sulfuric acid. In this article, we will discuss polyatomic ions. Table \(\pageindex{1}\) lists the ion. 2al(oh)3 + 3h2so4 → al2(so4)3 + 6h2o. The cation is written first. Aluminium sulfate is made by adding aluminium hydroxide i.e., al(oh)3, to sulfuric acid i.e., h2so4. For example, no 3 − is the. . Aluminum Sulfate Polyatomic Ion.