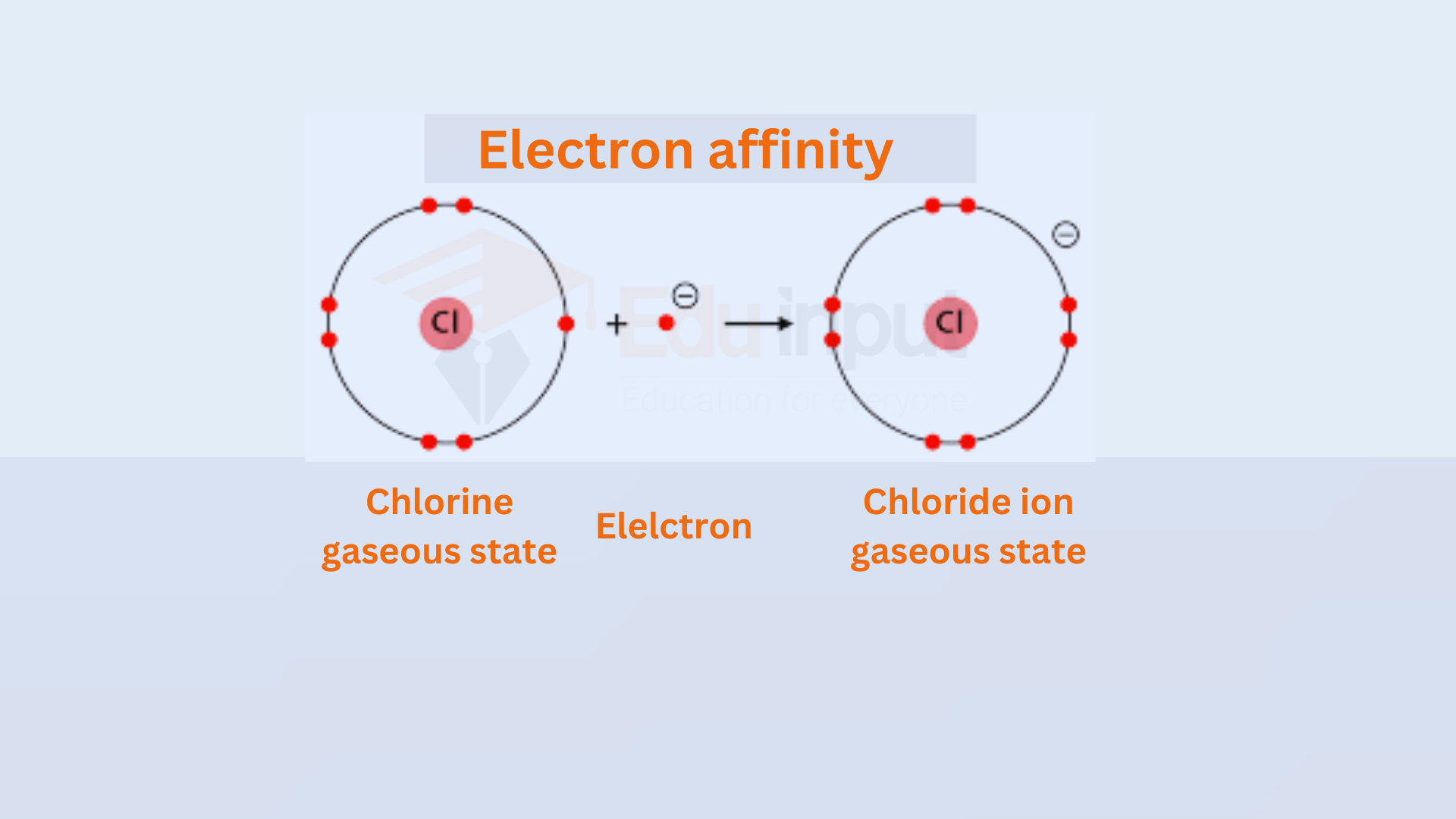
Chlorine Bromine Electron Affinity . So, why does that happen? The negative sign indicates a release of energy. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. as the name suggests, electron affinity is the ability of an atom to accept an electron. As you know, both chlorine and bromine are located in group 17 of the. chlorine most strongly attracts extra electrons; Neon most weakly attracts an extra electron. The first electron affinities of the group 7 elements electron affinity is defined as the energy released when an electron is added to a gaseous atom or ion. The electron affinities of the noble gases. By convention, the negative sign shows a release of energy. chlorine does have a higher electron affinity than bromine. for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: Unlike electronegativity, electron affinity is a.
from ar.inspiredpencil.com
Unlike electronegativity, electron affinity is a. chlorine most strongly attracts extra electrons; As you know, both chlorine and bromine are located in group 17 of the. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron affinity is defined as the energy released when an electron is added to a gaseous atom or ion. as the name suggests, electron affinity is the ability of an atom to accept an electron. The electron affinities of the noble gases. So, why does that happen? The negative sign indicates a release of energy. By convention, the negative sign shows a release of energy.
Electron Affinity Diagram
Chlorine Bromine Electron Affinity for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: electron affinity is defined as the energy released when an electron is added to a gaseous atom or ion. chlorine most strongly attracts extra electrons; 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. Unlike electronegativity, electron affinity is a. for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: By convention, the negative sign shows a release of energy. chlorine does have a higher electron affinity than bromine. The negative sign indicates a release of energy. The first electron affinities of the group 7 elements as the name suggests, electron affinity is the ability of an atom to accept an electron. So, why does that happen? As you know, both chlorine and bromine are located in group 17 of the. Neon most weakly attracts an extra electron. The electron affinities of the noble gases.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Bromine Electron Affinity chlorine most strongly attracts extra electrons; So, why does that happen? Neon most weakly attracts an extra electron. The first electron affinities of the group 7 elements as the name suggests, electron affinity is the ability of an atom to accept an electron. Unlike electronegativity, electron affinity is a. The electron affinities of the noble gases. electron. Chlorine Bromine Electron Affinity.
From ar.inspiredpencil.com
Electron Affinity Graph Chlorine Bromine Electron Affinity as the name suggests, electron affinity is the ability of an atom to accept an electron. As you know, both chlorine and bromine are located in group 17 of the. The negative sign indicates a release of energy. chlorine most strongly attracts extra electrons; Neon most weakly attracts an extra electron. chlorine does have a higher electron. Chlorine Bromine Electron Affinity.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b Chlorine Bromine Electron Affinity chlorine does have a higher electron affinity than bromine. By convention, the negative sign shows a release of energy. electron affinity is defined as the energy released when an electron is added to a gaseous atom or ion. So, why does that happen? The negative sign indicates a release of energy. As you know, both chlorine and bromine. Chlorine Bromine Electron Affinity.
From www.vedantu.com
Going from fluorine, chlorine, bromine to iodine the electronegativity Chlorine Bromine Electron Affinity As you know, both chlorine and bromine are located in group 17 of the. as the name suggests, electron affinity is the ability of an atom to accept an electron. for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: chlorine most strongly attracts extra electrons; Neon most weakly attracts an. Chlorine Bromine Electron Affinity.
From cewldcrl.blob.core.windows.net
Does Chlorine Have Electron Affinity at Holly Hauck blog Chlorine Bromine Electron Affinity 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. chlorine most strongly attracts extra electrons; By convention, the negative sign shows a release of energy. as the name suggests, electron affinity is the ability of an atom to accept an electron. As you. Chlorine Bromine Electron Affinity.
From byjus.com
6. d the electron affinity of chlorine from the following data Chlorine Bromine Electron Affinity So, why does that happen? Neon most weakly attracts an extra electron. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. The electron affinities of the noble gases. chlorine most strongly attracts extra electrons; Unlike electronegativity, electron affinity is a. for example, chlorine. Chlorine Bromine Electron Affinity.
From www.kingsleygateuat.com
Commission wants checkout and appeal lodge above email at take Chlorine Bromine Electron Affinity The negative sign indicates a release of energy. The first electron affinities of the group 7 elements The electron affinities of the noble gases. Unlike electronegativity, electron affinity is a. chlorine does have a higher electron affinity than bromine. So, why does that happen? By convention, the negative sign shows a release of energy. 125 rows the electron. Chlorine Bromine Electron Affinity.
From chemistry.stackexchange.com
chemistry If Oxygen has a lower electron affinity than Chlorine Bromine Electron Affinity The first electron affinities of the group 7 elements Unlike electronegativity, electron affinity is a. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. By convention, the negative sign shows a release of energy. Neon most weakly attracts an extra electron. electron affinity is. Chlorine Bromine Electron Affinity.
From resource.studiaacademy.com
Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Chlorine Bromine Electron Affinity The electron affinities of the noble gases. So, why does that happen? Unlike electronegativity, electron affinity is a. electron affinity is defined as the energy released when an electron is added to a gaseous atom or ion. for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: As you know, both chlorine. Chlorine Bromine Electron Affinity.
From www.chegg.com
Solved Electron Affinity Chlorine Flourine Bromine Electron Chlorine Bromine Electron Affinity 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. The electron affinities of the noble gases. The negative sign indicates a release of energy. The first electron affinities of the group 7 elements Unlike electronegativity, electron affinity is a. As you know, both chlorine and. Chlorine Bromine Electron Affinity.
From www.youtube.com
Fluorine Has Less Electron Gain Enthalpy Than Chlorine Periodic table Chlorine Bromine Electron Affinity as the name suggests, electron affinity is the ability of an atom to accept an electron. Unlike electronegativity, electron affinity is a. Neon most weakly attracts an extra electron. chlorine most strongly attracts extra electrons; chlorine does have a higher electron affinity than bromine. 125 rows the electron affinity is defined as the amount of energy. Chlorine Bromine Electron Affinity.
From www.youtube.com
The electron gain enthalpy (in \( \mathrm{kJ} / \mathrm{mol} \) ) of Chlorine Bromine Electron Affinity Neon most weakly attracts an extra electron. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. So, why does that happen? The first electron affinities of the group 7 elements The negative sign indicates a release of energy. electron affinity is defined as the. Chlorine Bromine Electron Affinity.
From www.pinterest.com
Periodic Table Electronegativity Trend Ionization energy Chlorine Bromine Electron Affinity The negative sign indicates a release of energy. The electron affinities of the noble gases. The first electron affinities of the group 7 elements electron affinity is defined as the energy released when an electron is added to a gaseous atom or ion. for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to. Chlorine Bromine Electron Affinity.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Chlorine Bromine Electron Affinity as the name suggests, electron affinity is the ability of an atom to accept an electron. By convention, the negative sign shows a release of energy. The electron affinities of the noble gases. electron affinity is defined as the energy released when an electron is added to a gaseous atom or ion. 125 rows the electron affinity. Chlorine Bromine Electron Affinity.
From pediaa.com
Difference Between Bromine and Chlorine Chlorine Bromine Electron Affinity Unlike electronegativity, electron affinity is a. chlorine most strongly attracts extra electrons; chlorine does have a higher electron affinity than bromine. electron affinity is defined as the energy released when an electron is added to a gaseous atom or ion. The negative sign indicates a release of energy. The electron affinities of the noble gases. for. Chlorine Bromine Electron Affinity.
From www.nagwa.com
Question Video Determining the Equation that Represents the Electron Chlorine Bromine Electron Affinity chlorine most strongly attracts extra electrons; The electron affinities of the noble gases. as the name suggests, electron affinity is the ability of an atom to accept an electron. By convention, the negative sign shows a release of energy. So, why does that happen? The first electron affinities of the group 7 elements chlorine does have a. Chlorine Bromine Electron Affinity.
From wiredataspremajte29.z14.web.core.windows.net
How To Calculate Born Haber Cycle Chlorine Bromine Electron Affinity As you know, both chlorine and bromine are located in group 17 of the. chlorine does have a higher electron affinity than bromine. The electron affinities of the noble gases. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. The first electron affinities of. Chlorine Bromine Electron Affinity.
From cewldcrl.blob.core.windows.net
Does Chlorine Have Electron Affinity at Holly Hauck blog Chlorine Bromine Electron Affinity as the name suggests, electron affinity is the ability of an atom to accept an electron. for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: chlorine does have a higher electron affinity than bromine. So, why does that happen? chlorine most strongly attracts extra electrons; Unlike electronegativity, electron affinity. Chlorine Bromine Electron Affinity.
From periodictableguide.com
Electron Affinity Chart (Labeled Periodic table + List) Chlorine Bromine Electron Affinity 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. as the name suggests, electron affinity is the ability of an atom to accept an electron. The negative sign indicates a release of energy. electron affinity is defined as the energy released when an. Chlorine Bromine Electron Affinity.
From ecurrencythailand.com
Which Of The Following Has Highest Electron Affinity Of Fluorine Chlorine Bromine Electron Affinity for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: By convention, the negative sign shows a release of energy. The electron affinities of the noble gases. chlorine does have a higher electron affinity than bromine. electron affinity is defined as the energy released when an electron is added to a. Chlorine Bromine Electron Affinity.
From chemistry.stackexchange.com
periodic trends If fluorine has a lower electron affinity than Chlorine Bromine Electron Affinity chlorine most strongly attracts extra electrons; for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: Neon most weakly attracts an extra electron. chlorine does have a higher electron affinity than bromine. So, why does that happen? As you know, both chlorine and bromine are located in group 17 of the.. Chlorine Bromine Electron Affinity.
From www.youtube.com
Electron Affinity Trend, Basic Introduction, Chemistry YouTube Chlorine Bromine Electron Affinity Neon most weakly attracts an extra electron. So, why does that happen? 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. chlorine does have a higher electron affinity than bromine. as the name suggests, electron affinity is the ability of an atom to. Chlorine Bromine Electron Affinity.
From www.youtube.com
3.2 Electron affinity (SL) YouTube Chlorine Bromine Electron Affinity The negative sign indicates a release of energy. Neon most weakly attracts an extra electron. As you know, both chlorine and bromine are located in group 17 of the. chlorine most strongly attracts extra electrons; chlorine does have a higher electron affinity than bromine. as the name suggests, electron affinity is the ability of an atom to. Chlorine Bromine Electron Affinity.
From www.numerade.com
SOLVEDThe element having highest electron affinity is (a) bromine (b Chlorine Bromine Electron Affinity electron affinity is defined as the energy released when an electron is added to a gaseous atom or ion. Neon most weakly attracts an extra electron. So, why does that happen? The negative sign indicates a release of energy. By convention, the negative sign shows a release of energy. for example, chlorine can oxidise the bromide ions (in,. Chlorine Bromine Electron Affinity.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation Chlorine Bromine Electron Affinity So, why does that happen? The negative sign indicates a release of energy. chlorine most strongly attracts extra electrons; By convention, the negative sign shows a release of energy. electron affinity is defined as the energy released when an electron is added to a gaseous atom or ion. The first electron affinities of the group 7 elements . Chlorine Bromine Electron Affinity.
From www.doubtnut.com
The electron affinity of bromine atom is equal to the..........of brom Chlorine Bromine Electron Affinity for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: Unlike electronegativity, electron affinity is a. The first electron affinities of the group 7 elements As you know, both chlorine and bromine are located in group 17 of the. Neon most weakly attracts an extra electron. as the name suggests, electron affinity. Chlorine Bromine Electron Affinity.
From www.youtube.com
Halogens second electron Affinity is zero Why?Fluorine chlorine bromine Chlorine Bromine Electron Affinity chlorine most strongly attracts extra electrons; So, why does that happen? electron affinity is defined as the energy released when an electron is added to a gaseous atom or ion. chlorine does have a higher electron affinity than bromine. The first electron affinities of the group 7 elements Neon most weakly attracts an extra electron. The negative. Chlorine Bromine Electron Affinity.
From www.numerade.com
SOLVEDUse electron configurations to explain why (a) sulfur has a Chlorine Bromine Electron Affinity for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: Unlike electronegativity, electron affinity is a. By convention, the negative sign shows a release of energy. electron affinity is defined as the energy released when an electron is added to a gaseous atom or ion. The first electron affinities of the group. Chlorine Bromine Electron Affinity.
From ecurrencythailand.com
Which Of The Following Has Highest Electron Affinity Of Fluorine Chlorine Bromine Electron Affinity Unlike electronegativity, electron affinity is a. The negative sign indicates a release of energy. as the name suggests, electron affinity is the ability of an atom to accept an electron. for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: The first electron affinities of the group 7 elements chlorine most. Chlorine Bromine Electron Affinity.
From exorwrmka.blob.core.windows.net
Bromine Of Electron Affinity at Curtis Phillips blog Chlorine Bromine Electron Affinity for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: chlorine does have a higher electron affinity than bromine. Unlike electronegativity, electron affinity is a. The first electron affinities of the group 7 elements 125 rows the electron affinity is defined as the amount of energy released when an electron is. Chlorine Bromine Electron Affinity.
From ar.inspiredpencil.com
Electron Affinity Diagram Chlorine Bromine Electron Affinity The electron affinities of the noble gases. By convention, the negative sign shows a release of energy. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. As you know, both chlorine and bromine are located in group 17 of the. electron affinity is defined. Chlorine Bromine Electron Affinity.
From www.toppr.com
Match the following elements of column I with their property g Column 1 Chlorine Bromine Electron Affinity chlorine most strongly attracts extra electrons; So, why does that happen? Neon most weakly attracts an extra electron. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. Unlike electronegativity, electron affinity is a. By convention, the negative sign shows a release of energy. . Chlorine Bromine Electron Affinity.
From www.numerade.com
SOLVED Does chlorine or bromine have a more negative (more favorable Chlorine Bromine Electron Affinity 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. As you know, both chlorine and bromine are located in group 17 of the. Neon most weakly attracts an extra electron. as the name suggests, electron affinity is the ability of an atom to accept. Chlorine Bromine Electron Affinity.
From schematicdatavenin77.z5.web.core.windows.net
Lewis Electron Dot Symbol For Bromine Chlorine Bromine Electron Affinity Unlike electronegativity, electron affinity is a. So, why does that happen? The electron affinities of the noble gases. chlorine does have a higher electron affinity than bromine. The first electron affinities of the group 7 elements for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: As you know, both chlorine and. Chlorine Bromine Electron Affinity.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Chlorine Bromine Electron Affinity So, why does that happen? as the name suggests, electron affinity is the ability of an atom to accept an electron. The first electron affinities of the group 7 elements chlorine does have a higher electron affinity than bromine. Neon most weakly attracts an extra electron. By convention, the negative sign shows a release of energy. 125. Chlorine Bromine Electron Affinity.