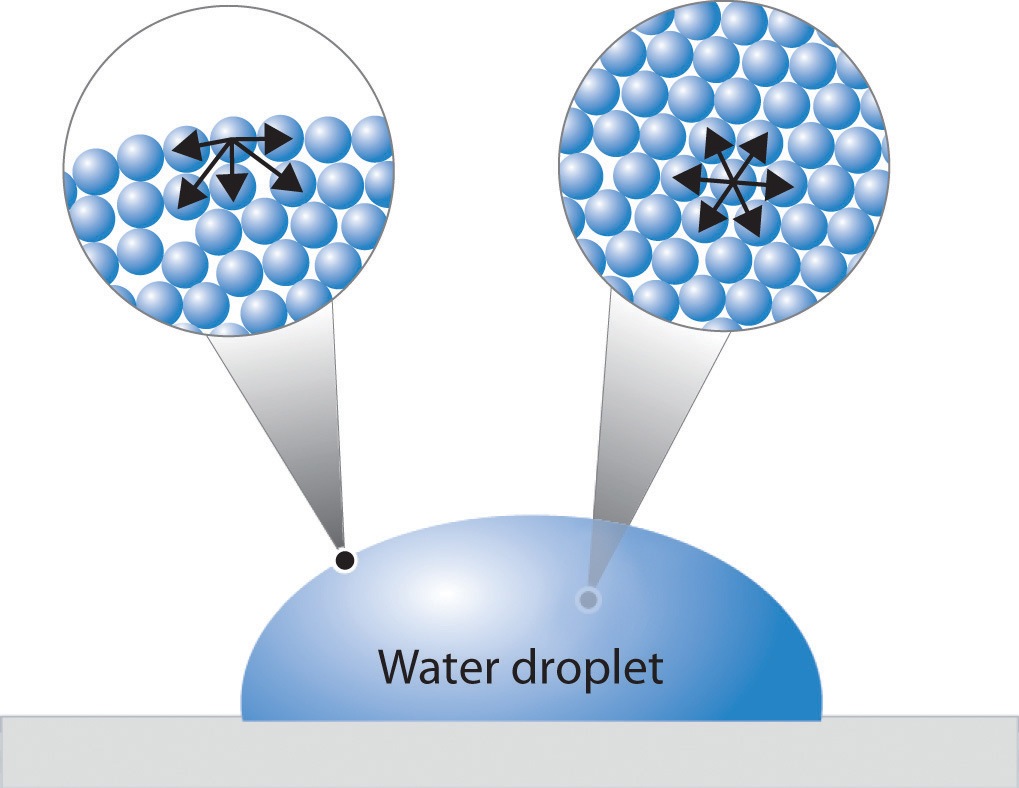
What Does Reducing Surface Tension Mean . surface tension is a physical property of liquids that refers to the force acting on the surface of the liquid and which tends to reduce the surface area to the. surfactants are great at lowering surface tension by changing the molecular composition of liquid surfaces, but they can also alter the composition of surfaces. Water has a surface tension of 0.07275 joule per square metre at 20. surfactants are compounds that lower the surface tension of a liquid like water, the interfacial tension between two liquids,. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the. surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that can lower the surface tension of water. surface tension may be expressed, therefore, in units of energy per unit area (square metres).
from chem.libretexts.org
surfactants are great at lowering surface tension by changing the molecular composition of liquid surfaces, but they can also alter the composition of surfaces. Since these intermolecular forces vary depending on the. Water has a surface tension of 0.07275 joule per square metre at 20. surface tension is a physical property of liquids that refers to the force acting on the surface of the liquid and which tends to reduce the surface area to the. surface tension may be expressed, therefore, in units of energy per unit area (square metres). surfactants are compounds that lower the surface tension of a liquid like water, the interfacial tension between two liquids,. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that can lower the surface tension of water.
Surface Tension Chemistry LibreTexts
What Does Reducing Surface Tension Mean surface tension may be expressed, therefore, in units of energy per unit area (square metres). surfactants are great at lowering surface tension by changing the molecular composition of liquid surfaces, but they can also alter the composition of surfaces. surfactants are compounds that lower the surface tension of a liquid like water, the interfacial tension between two liquids,. surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that can lower the surface tension of water. Since these intermolecular forces vary depending on the. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension may be expressed, therefore, in units of energy per unit area (square metres). Water has a surface tension of 0.07275 joule per square metre at 20. surface tension is a physical property of liquids that refers to the force acting on the surface of the liquid and which tends to reduce the surface area to the.
From www.youtube.com
Surfactant and Surface Tension in Respiration Breathing Mechanics What Does Reducing Surface Tension Mean surface tension is a physical property of liquids that refers to the force acting on the surface of the liquid and which tends to reduce the surface area to the. surfactants are great at lowering surface tension by changing the molecular composition of liquid surfaces, but they can also alter the composition of surfaces. surface tension is. What Does Reducing Surface Tension Mean.
From hubpages.com
Surfactant Lowering Pulmonary Surface Tension Owlcation What Does Reducing Surface Tension Mean surfactants are compounds that lower the surface tension of a liquid like water, the interfacial tension between two liquids,. surface tension may be expressed, therefore, in units of energy per unit area (square metres). Since these intermolecular forces vary depending on the. surface tension is a crucial property of a liquid that allows it to resist being. What Does Reducing Surface Tension Mean.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog What Does Reducing Surface Tension Mean Since these intermolecular forces vary depending on the. surfactants are great at lowering surface tension by changing the molecular composition of liquid surfaces, but they can also alter the composition of surfaces. surface tension may be expressed, therefore, in units of energy per unit area (square metres). surface tension is a crucial property of a liquid that. What Does Reducing Surface Tension Mean.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 What Does Reducing Surface Tension Mean surfactants are compounds that lower the surface tension of a liquid like water, the interfacial tension between two liquids,. surfactants are great at lowering surface tension by changing the molecular composition of liquid surfaces, but they can also alter the composition of surfaces. Water has a surface tension of 0.07275 joule per square metre at 20. surface. What Does Reducing Surface Tension Mean.
From lessonschoolcarpino.z21.web.core.windows.net
How To Explain Surface Tension What Does Reducing Surface Tension Mean surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension may be expressed, therefore, in units of energy per unit area (square metres). Since these intermolecular forces vary depending on the. Water has a surface tension of 0.07275 joule per square metre at 20. surfactants. What Does Reducing Surface Tension Mean.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint What Does Reducing Surface Tension Mean surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that can lower the surface tension of water. surface tension is a physical property. What Does Reducing Surface Tension Mean.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint What Does Reducing Surface Tension Mean surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that can lower the surface tension of water. surfactants are great at lowering surface tension by changing the molecular composition of liquid surfaces, but they can also alter the composition of surfaces. surface tension. What Does Reducing Surface Tension Mean.
From www.instructables.com
Surface TensionThrough Expirements 4 Steps Instructables What Does Reducing Surface Tension Mean surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that can lower the surface tension of water. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a surface tension of 0.07275. What Does Reducing Surface Tension Mean.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector What Does Reducing Surface Tension Mean surfactants are compounds that lower the surface tension of a liquid like water, the interfacial tension between two liquids,. Water has a surface tension of 0.07275 joule per square metre at 20. surface tension is a physical property of liquids that refers to the force acting on the surface of the liquid and which tends to reduce the. What Does Reducing Surface Tension Mean.
From www.youtube.com
Surface Tension of Water Explained YouTube What Does Reducing Surface Tension Mean Since these intermolecular forces vary depending on the. surface tension may be expressed, therefore, in units of energy per unit area (square metres). surface tension is a physical property of liquids that refers to the force acting on the surface of the liquid and which tends to reduce the surface area to the. surfactants are compounds that. What Does Reducing Surface Tension Mean.
From exoxqkwlq.blob.core.windows.net
How Does Surface Tension Relate To Water at Ron Lowery blog What Does Reducing Surface Tension Mean surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that can lower the surface tension of water. surfactants are great at lowering surface tension by changing the molecular composition of liquid surfaces, but they can also alter the composition of surfaces. Since these intermolecular. What Does Reducing Surface Tension Mean.
From www.moleaer.com
How nanobubbles improve water infiltration by reducing surface tension What Does Reducing Surface Tension Mean surfactants are compounds that lower the surface tension of a liquid like water, the interfacial tension between two liquids,. Water has a surface tension of 0.07275 joule per square metre at 20. surface tension is a physical property of liquids that refers to the force acting on the surface of the liquid and which tends to reduce the. What Does Reducing Surface Tension Mean.
From www.moleaer.com
How nanobubbles improve water infiltration by reducing surface tension What Does Reducing Surface Tension Mean surface tension is a physical property of liquids that refers to the force acting on the surface of the liquid and which tends to reduce the surface area to the. surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that can lower the surface. What Does Reducing Surface Tension Mean.
From owlcation.com
Surfactant Lowering Pulmonary Surface Tension Owlcation What Does Reducing Surface Tension Mean surfactants are great at lowering surface tension by changing the molecular composition of liquid surfaces, but they can also alter the composition of surfaces. Since these intermolecular forces vary depending on the. surface tension is a physical property of liquids that refers to the force acting on the surface of the liquid and which tends to reduce the. What Does Reducing Surface Tension Mean.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical What Does Reducing Surface Tension Mean Since these intermolecular forces vary depending on the. Water has a surface tension of 0.07275 joule per square metre at 20. surfactants are compounds that lower the surface tension of a liquid like water, the interfacial tension between two liquids,. surfactants are great at lowering surface tension by changing the molecular composition of liquid surfaces, but they can. What Does Reducing Surface Tension Mean.
From www.researchgate.net
Surface tension decreases with increasing the surfactant concentration What Does Reducing Surface Tension Mean surfactants are great at lowering surface tension by changing the molecular composition of liquid surfaces, but they can also alter the composition of surfaces. surface tension may be expressed, therefore, in units of energy per unit area (square metres). surface tension is the energy, or work, required to increase the surface area of a liquid due to. What Does Reducing Surface Tension Mean.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance What Does Reducing Surface Tension Mean surfactants are compounds that lower the surface tension of a liquid like water, the interfacial tension between two liquids,. surface tension is a physical property of liquids that refers to the force acting on the surface of the liquid and which tends to reduce the surface area to the. surface tension is the energy, or work, required. What Does Reducing Surface Tension Mean.
From userdatasynoecizes.z5.web.core.windows.net
Explain The Concept Of Surface Tension What Does Reducing Surface Tension Mean surfactants are compounds that lower the surface tension of a liquid like water, the interfacial tension between two liquids,. surfactants are great at lowering surface tension by changing the molecular composition of liquid surfaces, but they can also alter the composition of surfaces. surface tension is a physical property of liquids that refers to the force acting. What Does Reducing Surface Tension Mean.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts What Does Reducing Surface Tension Mean surface tension may be expressed, therefore, in units of energy per unit area (square metres). surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that can lower the surface tension of water. Water has a surface tension of 0.07275 joule per square metre at. What Does Reducing Surface Tension Mean.
From circuitenginemeseta101.z22.web.core.windows.net
Diagram Of Surface Tension What Does Reducing Surface Tension Mean surfactants are great at lowering surface tension by changing the molecular composition of liquid surfaces, but they can also alter the composition of surfaces. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the. surfactants are compounds that lower. What Does Reducing Surface Tension Mean.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs What Does Reducing Surface Tension Mean surfactants are great at lowering surface tension by changing the molecular composition of liquid surfaces, but they can also alter the composition of surfaces. surface tension is a physical property of liquids that refers to the force acting on the surface of the liquid and which tends to reduce the surface area to the. surface tension may. What Does Reducing Surface Tension Mean.
From chemistry.stackexchange.com
aqueous solution How can proteins reduce surface tension? Chemistry What Does Reducing Surface Tension Mean surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surfactants are compounds that lower the surface tension of a liquid like water, the interfacial tension between two liquids,. surface tension is a physical property of liquids that refers to the force acting on the surface of. What Does Reducing Surface Tension Mean.
From userdatasynoecizes.z5.web.core.windows.net
Explain The Concept Of Surface Tension What Does Reducing Surface Tension Mean surfactants are great at lowering surface tension by changing the molecular composition of liquid surfaces, but they can also alter the composition of surfaces. surfactants are compounds that lower the surface tension of a liquid like water, the interfacial tension between two liquids,. surface tension may be expressed, therefore, in units of energy per unit area (square. What Does Reducing Surface Tension Mean.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit What Does Reducing Surface Tension Mean surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that can lower the surface tension of water. Water has a surface tension of 0.07275 joule per square metre at 20. surfactants are great at lowering surface tension by changing the molecular composition of liquid. What Does Reducing Surface Tension Mean.
From www.youtube.com
How does Surface Tension work? YouTube What Does Reducing Surface Tension Mean surfactants are compounds that lower the surface tension of a liquid like water, the interfacial tension between two liquids,. surfactants are great at lowering surface tension by changing the molecular composition of liquid surfaces, but they can also alter the composition of surfaces. surface tension is a crucial property of a liquid that allows it to resist. What Does Reducing Surface Tension Mean.
From thechemistrynotes.com
Surface Tension Definition, Formula, Unit, Causes, Examples, Consequences What Does Reducing Surface Tension Mean Since these intermolecular forces vary depending on the. Water has a surface tension of 0.07275 joule per square metre at 20. surfactants are great at lowering surface tension by changing the molecular composition of liquid surfaces, but they can also alter the composition of surfaces. surface tension is a crucial property of a liquid that allows it to. What Does Reducing Surface Tension Mean.
From cexrwxpx.blob.core.windows.net
How To Find Lowest Surface Tension at William Ross blog What Does Reducing Surface Tension Mean Water has a surface tension of 0.07275 joule per square metre at 20. surfactants are great at lowering surface tension by changing the molecular composition of liquid surfaces, but they can also alter the composition of surfaces. surface tension may be expressed, therefore, in units of energy per unit area (square metres). surfactants are compounds that lower. What Does Reducing Surface Tension Mean.
From www.slideserve.com
PPT Ch. 2 States of Matter PowerPoint Presentation, free download What Does Reducing Surface Tension Mean Water has a surface tension of 0.07275 joule per square metre at 20. surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that can lower the surface tension of water. Since these intermolecular forces vary depending on the. surface tension is a physical property. What Does Reducing Surface Tension Mean.
From www.vrogue.co
Surfactant Lowering Pulmonary Surface Tension Owlcati vrogue.co What Does Reducing Surface Tension Mean surface tension is a physical property of liquids that refers to the force acting on the surface of the liquid and which tends to reduce the surface area to the. Water has a surface tension of 0.07275 joule per square metre at 20. surface tension is a crucial property of a liquid that allows it to resist being. What Does Reducing Surface Tension Mean.
From delprofedefisica.blogspot.com
Física Tensión superficial What Does Reducing Surface Tension Mean surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that can lower the surface tension of water. surface tension is a physical property of liquids that refers to the force acting on the surface of the liquid and which tends to reduce the surface. What Does Reducing Surface Tension Mean.
From www.lecturio.com
Ventilación Mecánica de la Respiración Concise Medical Knowledge What Does Reducing Surface Tension Mean surface tension is a physical property of liquids that refers to the force acting on the surface of the liquid and which tends to reduce the surface area to the. Since these intermolecular forces vary depending on the. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.. What Does Reducing Surface Tension Mean.
From owlcation.com
Surfactant Lowering Pulmonary Surface Tension Owlcation What Does Reducing Surface Tension Mean Water has a surface tension of 0.07275 joule per square metre at 20. surface tension may be expressed, therefore, in units of energy per unit area (square metres). Since these intermolecular forces vary depending on the. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surfactants. What Does Reducing Surface Tension Mean.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID What Does Reducing Surface Tension Mean surface tension may be expressed, therefore, in units of energy per unit area (square metres). Water has a surface tension of 0.07275 joule per square metre at 20. surfactants are great at lowering surface tension by changing the molecular composition of liquid surfaces, but they can also alter the composition of surfaces. surface tension is the energy,. What Does Reducing Surface Tension Mean.
From lessonlibnanometres.z13.web.core.windows.net
Surface Tension Simple Explanation What Does Reducing Surface Tension Mean Water has a surface tension of 0.07275 joule per square metre at 20. surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that can lower the surface tension of water. surface tension may be expressed, therefore, in units of energy per unit area (square. What Does Reducing Surface Tension Mean.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID3227401 What Does Reducing Surface Tension Mean surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that can lower the surface tension of water. Water has a surface tension of 0.07275 joule per square metre at 20. Since these intermolecular forces vary depending on the. surfactants are compounds that lower the. What Does Reducing Surface Tension Mean.