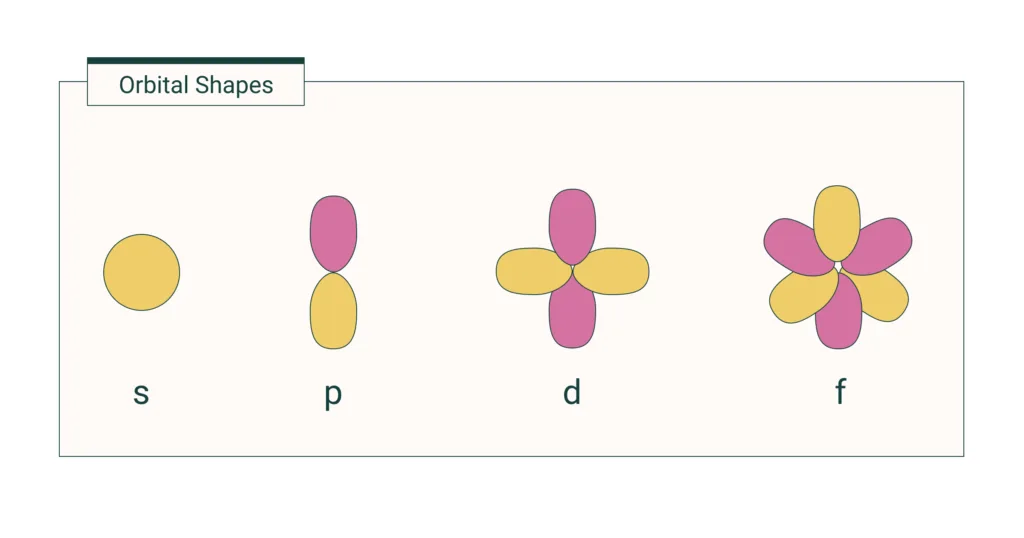
What Is The Meaning Of S P D F . the orbital names s, p, d, and f describe electron configuration. These line groups are called sharp, principal, diffuse, and fundamental. At the first energy level, the only orbital. The orbitals in an atom are organized into different layers or electron shells. These line groups are called sharp, principal, diffuse, and fundamental. The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle. periodic table blocks are sets of elements grouped by their valence electron orbitals. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). what does s, p, d, f stand for? ⚛ each of the subshells. Each block indicates which electron sublevel is in the process of being filled. ⚛ electrons making up an atom are arranged in subshells. The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the spectra of the alkali metals. (1) ⚛ there are four types of subshells. the simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively.
from h-o-m-e.org
The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the spectra of the alkali metals. Each block indicates which electron sublevel is in the process of being filled. the simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle. ⚛ each of the subshells. ⚛ electrons making up an atom are arranged in subshells. These line groups are called sharp, principal, diffuse, and fundamental. (1) ⚛ there are four types of subshells. At the first energy level, the only orbital. periodic table blocks are sets of elements grouped by their valence electron orbitals.
Chemistry Sublevels An Introduction to s, p, d, and f Sublevels
What Is The Meaning Of S P D F the simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. Each block indicates which electron sublevel is in the process of being filled. These line groups are called sharp, principal, diffuse, and fundamental. ⚛ electrons making up an atom are arranged in subshells. These line groups are called sharp, principal, diffuse, and fundamental. the orbital names s, p, d, and f describe electron configuration. At the first energy level, the only orbital. The orbitals in an atom are organized into different layers or electron shells. the simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the spectra of the alkali metals. what does s, p, d, f stand for? (1) ⚛ there are four types of subshells. periodic table blocks are sets of elements grouped by their valence electron orbitals. The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). ⚛ each of the subshells.
From byjus.com
What is s, p, d, f, What Is The Meaning Of S P D F These line groups are called sharp, principal, diffuse, and fundamental. the simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. what does s, p, d, f stand for? These line groups are called sharp, principal, diffuse, and fundamental. At the. What Is The Meaning Of S P D F.
From stock.adobe.com
Periodic table. s,p,d,f block elements. Electron configuration What Is The Meaning Of S P D F (1) ⚛ there are four types of subshells. ⚛ each of the subshells. The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the spectra of the alkali metals. periodic table blocks are sets of elements grouped by their valence electron orbitals. what does s, p, d, f stand. What Is The Meaning Of S P D F.
From www.vrogue.co
Shapes Of Orbitals S P D Shapes vrogue.co What Is The Meaning Of S P D F Each block indicates which electron sublevel is in the process of being filled. the simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. These line groups are called sharp, principal, diffuse, and fundamental. periodic table blocks are sets of elements. What Is The Meaning Of S P D F.
From bobbyversechmx.blogspot.com
Bobbyverse! Orbitales atómicos What Is The Meaning Of S P D F The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle. what does s, p, d, f stand for? Each block indicates which electron sublevel is in the process of being filled. The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the spectra of. What Is The Meaning Of S P D F.
From utedzz.blogspot.com
Periodic Table Showing S P D F Blocks Periodic Table Timeline What Is The Meaning Of S P D F Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). At the first energy level, the only orbital. periodic table blocks are sets of elements grouped by their valence electron orbitals. These line groups are called sharp, principal, diffuse, and fundamental. Each block indicates which electron sublevel is in the process of being filled.. What Is The Meaning Of S P D F.
From manualworshipped.z14.web.core.windows.net
Orbital Diagram S P D F What Is The Meaning Of S P D F periodic table blocks are sets of elements grouped by their valence electron orbitals. the simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. ⚛ each of the subshells. These line groups are called sharp, principal, diffuse, and fundamental. Each block. What Is The Meaning Of S P D F.
From www.pinterest.com
What Do S, P, D, and F Mean in Chemistry, and Why Do You Need Them What Is The Meaning Of S P D F the simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. ⚛ electrons making up an atom are arranged in subshells. periodic table blocks are sets of elements grouped by their valence electron orbitals. (1) ⚛ there are four types of. What Is The Meaning Of S P D F.
From ecurrencythailand.com
What Is F On The Periodic Table? The 12 Correct Answer What Is The Meaning Of S P D F At the first energy level, the only orbital. the orbital names s, p, d, and f describe electron configuration. The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle. (1) ⚛ there are four types of subshells. These line groups are called sharp, principal, diffuse, and fundamental. These line groups are called sharp,. What Is The Meaning Of S P D F.
From www.vrogue.co
S P D And F Block Elements Classification Of Elements vrogue.co What Is The Meaning Of S P D F what does s, p, d, f stand for? the orbital names s, p, d, and f describe electron configuration. These line groups are called sharp, principal, diffuse, and fundamental. The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the spectra of the alkali metals. ⚛ each of the. What Is The Meaning Of S P D F.
From www.youtube.com
Properties of s, p, d & f block YouTube What Is The Meaning Of S P D F The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle. Each block indicates which electron sublevel is in the process of being filled. (1) ⚛ there are four types of subshells. The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the spectra of the. What Is The Meaning Of S P D F.
From chemwiki.ucdavis.edu
Electron Configuration of Transition Metals Chemwiki What Is The Meaning Of S P D F what does s, p, d, f stand for? (1) ⚛ there are four types of subshells. The orbitals in an atom are organized into different layers or electron shells. the simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. Each. What Is The Meaning Of S P D F.
From www.youtube.com
Electron Configuration What is it? What are S P D F subshells? How to What Is The Meaning Of S P D F what does s, p, d, f stand for? the orbital names s, p, d, and f describe electron configuration. (1) ⚛ there are four types of subshells. The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle. ⚛ electrons making up an atom are arranged in subshells. The orbital names s, p,. What Is The Meaning Of S P D F.
From beccaandstephaniechemisty.weebly.com
Sublevels (S, P, D, F) What Is The Meaning Of S P D F The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle. These line groups are called sharp, principal, diffuse, and fundamental. These line groups are called sharp, principal, diffuse, and fundamental. ⚛ each of the subshells. (1) ⚛ there are four types of subshells. the orbital names s, p, d, and f describe electron. What Is The Meaning Of S P D F.
From www.shutterstock.com
Atomic Orbitals S P D F 库存插图 1829554250 Shutterstock What Is The Meaning Of S P D F Each block indicates which electron sublevel is in the process of being filled. what does s, p, d, f stand for? ⚛ each of the subshells. ⚛ electrons making up an atom are arranged in subshells. the simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ =. What Is The Meaning Of S P D F.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps What Is The Meaning Of S P D F Each block indicates which electron sublevel is in the process of being filled. These line groups are called sharp, principal, diffuse, and fundamental. the orbital names s, p, d, and f describe electron configuration. The orbitals in an atom are organized into different layers or electron shells. At the first energy level, the only orbital. ⚛ each of the. What Is The Meaning Of S P D F.
From www.slideserve.com
PPT To learn about the shapes of the s, p and d orbitals PowerPoint What Is The Meaning Of S P D F the simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle. ⚛ each of the subshells. periodic table blocks are sets of elements grouped by. What Is The Meaning Of S P D F.
From slideplayer.com
Parts of Unit 4 part b Electrons and Periodic Behavior ppt download What Is The Meaning Of S P D F (1) ⚛ there are four types of subshells. what does s, p, d, f stand for? ⚛ each of the subshells. The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the spectra of the alkali metals. ⚛ electrons making up an atom are arranged in subshells. Each block indicates. What Is The Meaning Of S P D F.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps What Is The Meaning Of S P D F Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). The orbitals in an atom are organized into different layers or electron shells. what does s, p, d, f stand for? ⚛ electrons making up an atom are arranged in subshells. The orbital names s, p, d, and f stand for names given to. What Is The Meaning Of S P D F.
From courses.lumenlearning.com
6.3 Development of Quantum Theory Chemistry What Is The Meaning Of S P D F The orbitals in an atom are organized into different layers or electron shells. At the first energy level, the only orbital. periodic table blocks are sets of elements grouped by their valence electron orbitals. The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle. ⚛ electrons making up an atom are arranged in. What Is The Meaning Of S P D F.
From socratic.com
s,p,d,f Orbitals Chemistry Socratic What Is The Meaning Of S P D F At the first energy level, the only orbital. The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the spectra of the alkali metals. the orbital names s, p, d, and f describe electron configuration. Each block indicates which electron sublevel is in the process of being filled. These line. What Is The Meaning Of S P D F.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps What Is The Meaning Of S P D F These line groups are called sharp, principal, diffuse, and fundamental. ⚛ electrons making up an atom are arranged in subshells. (1) ⚛ there are four types of subshells. ⚛ each of the subshells. The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle. The orbitals in an atom are organized into different layers or. What Is The Meaning Of S P D F.
From byjus.com
What do s, p, d, f stand for? What Is The Meaning Of S P D F ⚛ electrons making up an atom are arranged in subshells. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). The orbitals in an atom are organized into different layers or electron shells. Each block indicates which electron sublevel is in the process of being filled. what does s, p, d, f stand for?. What Is The Meaning Of S P D F.
From byjus.com
What is the value of s,p,d.f? What Is The Meaning Of S P D F The orbitals in an atom are organized into different layers or electron shells. the orbital names s, p, d, and f describe electron configuration. ⚛ each of the subshells. the simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. The. What Is The Meaning Of S P D F.
From ar.inspiredpencil.com
S P D F Orbital What Is The Meaning Of S P D F These line groups are called sharp, principal, diffuse, and fundamental. periodic table blocks are sets of elements grouped by their valence electron orbitals. (1) ⚛ there are four types of subshells. The orbitals in an atom are organized into different layers or electron shells. Each block indicates which electron sublevel is in the process of being filled. Not all. What Is The Meaning Of S P D F.
From h-o-m-e.org
Chemistry Sublevels An Introduction to s, p, d, and f Sublevels What Is The Meaning Of S P D F The orbitals in an atom are organized into different layers or electron shells. periodic table blocks are sets of elements grouped by their valence electron orbitals. ⚛ each of the subshells. ⚛ electrons making up an atom are arranged in subshells. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). The fifth d. What Is The Meaning Of S P D F.
From www.slideserve.com
PPT Identify S, P, D, F PowerPoint Presentation, free download ID What Is The Meaning Of S P D F The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the spectra of the alkali metals. the orbital names s, p, d, and f describe electron configuration. ⚛ electrons making up an atom are arranged in subshells. These line groups are called sharp, principal, diffuse, and fundamental. the simple. What Is The Meaning Of S P D F.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry What Is The Meaning Of S P D F what does s, p, d, f stand for? the simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. ⚛ electrons making up an atom are arranged in subshells. periodic table blocks are sets of elements grouped by their valence. What Is The Meaning Of S P D F.
From s3.amazonaws.com
How many electrons can s p d f orbitals hold, s bravo art, back pain What Is The Meaning Of S P D F The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle. ⚛ each of the subshells. ⚛ electrons making up an atom are arranged in subshells. These line groups are called sharp, principal, diffuse, and fundamental. At the first energy level, the only orbital. Each block indicates which electron sublevel is in the process of. What Is The Meaning Of S P D F.
From ar.inspiredpencil.com
Electron Orbitals S P D F What Is The Meaning Of S P D F These line groups are called sharp, principal, diffuse, and fundamental. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). (1) ⚛ there are four types of subshells. The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle. ⚛ each of the subshells. ⚛ electrons making up an atom. What Is The Meaning Of S P D F.
From ar.inspiredpencil.com
Electron Shells S P D F What Is The Meaning Of S P D F The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the spectra of the alkali metals. ⚛ electrons making up an atom are arranged in subshells. (1) ⚛ there are four types of subshells. These line groups are called sharp, principal, diffuse, and fundamental. The fifth d orbital is shaped like. What Is The Meaning Of S P D F.
From lavelle.chem.ucla.edu
s,p,d,f orbital characteristics? CHEMISTRY COMMUNITY What Is The Meaning Of S P D F At the first energy level, the only orbital. (1) ⚛ there are four types of subshells. These line groups are called sharp, principal, diffuse, and fundamental. Each block indicates which electron sublevel is in the process of being filled. These line groups are called sharp, principal, diffuse, and fundamental. ⚛ each of the subshells. ⚛ electrons making up an atom. What Is The Meaning Of S P D F.
From ar.inspiredpencil.com
Electron Orbitals S P D F What Is The Meaning Of S P D F Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). Each block indicates which electron sublevel is in the process of being filled. The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle. These line groups are called sharp, principal, diffuse, and fundamental. These line groups are called sharp,. What Is The Meaning Of S P D F.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps What Is The Meaning Of S P D F Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). Each block indicates which electron sublevel is in the process of being filled. the orbital names s, p, d, and f describe electron configuration. At the first energy level, the only orbital. ⚛ each of the subshells. The fifth d orbital is shaped like. What Is The Meaning Of S P D F.
From gbu-taganskij.ru
Chemical Bonding Atomic Orbitals, Shapes, Hybridization, 42 OFF What Is The Meaning Of S P D F the simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. the orbital names s, p, d, and f describe electron configuration. (1) ⚛ there are four types of subshells. ⚛ each of the subshells. The fifth d orbital is shaped. What Is The Meaning Of S P D F.
From www.slideserve.com
PPT s p d f Orbitals PowerPoint Presentation, free download ID847092 What Is The Meaning Of S P D F Each block indicates which electron sublevel is in the process of being filled. (1) ⚛ there are four types of subshells. At the first energy level, the only orbital. These line groups are called sharp, principal, diffuse, and fundamental. the simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number. What Is The Meaning Of S P D F.