Synthesis Non Example Chemistry . The reactants may be elements or compounds, while the product is always a compound. General form of synthesis reactions. The general form of a synthesis reaction is: A + b → ab. The construction of complex and defined new molecules is a challenging and complicated. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. Synthesis is the production of chemical compounds by reaction from simpler materials. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. Chemists synthesize chemical compounds that occur in nature in order to gain a better understanding of their structures. A combination reaction, also known as a synthesis reaction, is a reaction in which two or more substances combine to form a single new substance.
from www.a-levelnotes.co.uk
A + b → ab. Chemists synthesize chemical compounds that occur in nature in order to gain a better understanding of their structures. The general form of a synthesis reaction is: The construction of complex and defined new molecules is a challenging and complicated. Synthesis is the production of chemical compounds by reaction from simpler materials. General form of synthesis reactions. A combination reaction, also known as a synthesis reaction, is a reaction in which two or more substances combine to form a single new substance. The reactants may be elements or compounds, while the product is always a compound. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product.
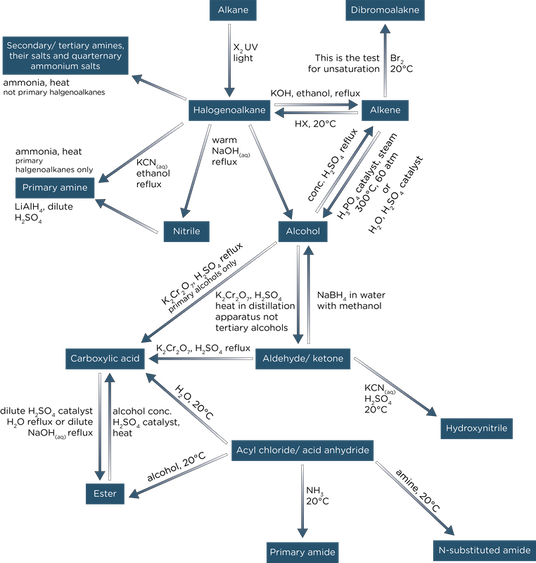
Alevel Chemistry AQA Notes Organic Synthesis (ALevel) ALEVEL NOTES
Synthesis Non Example Chemistry A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. Chemists synthesize chemical compounds that occur in nature in order to gain a better understanding of their structures. A + b → ab. The general form of a synthesis reaction is: Synthesis is the production of chemical compounds by reaction from simpler materials. General form of synthesis reactions. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. The reactants may be elements or compounds, while the product is always a compound. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. The construction of complex and defined new molecules is a challenging and complicated. A combination reaction, also known as a synthesis reaction, is a reaction in which two or more substances combine to form a single new substance.
From www.youtube.com
Synthesis Reactions YouTube Synthesis Non Example Chemistry General form of synthesis reactions. Chemists synthesize chemical compounds that occur in nature in order to gain a better understanding of their structures. The general form of a synthesis reaction is: Synthesis is the production of chemical compounds by reaction from simpler materials. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction.. Synthesis Non Example Chemistry.
From specificpolymers.com
What is Custom Synthesis & why you should outsource it? Synthesis Non Example Chemistry Chemists synthesize chemical compounds that occur in nature in order to gain a better understanding of their structures. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. The construction of complex and defined new molecules is a challenging and complicated. A +. Synthesis Non Example Chemistry.
From www.thoughtco.com
Synthesis Reaction Description Plus Examples Synthesis Non Example Chemistry A combination reaction, also known as a synthesis reaction, is a reaction in which two or more substances combine to form a single new substance. General form of synthesis reactions. Synthesis is the production of chemical compounds by reaction from simpler materials. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. Chemists. Synthesis Non Example Chemistry.
From www.masterorganicchemistry.com
Aromatic Synthesis Order of Reactions Master Organic Chemistry Synthesis Non Example Chemistry The reactants may be elements or compounds, while the product is always a compound. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. Synthesis is. Synthesis Non Example Chemistry.
From www.chegg.com
Organic Chemistry Synthesis practice Part 3 I Synthesis Non Example Chemistry The reactants may be elements or compounds, while the product is always a compound. A + b → ab. Chemists synthesize chemical compounds that occur in nature in order to gain a better understanding of their structures. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form. Synthesis Non Example Chemistry.
From colouremployer8.gitlab.io
Supreme 4 Types Of Chemical Reactions Reactants And Products Cellular Synthesis Non Example Chemistry A combination reaction, also known as a synthesis reaction, is a reaction in which two or more substances combine to form a single new substance. The construction of complex and defined new molecules is a challenging and complicated. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. The general form of a. Synthesis Non Example Chemistry.
From www.pinterest.com.mx
Synthesis (4) Alkene Reaction Map, Including Alkyl Halide Reactions Synthesis Non Example Chemistry Synthesis is the production of chemical compounds by reaction from simpler materials. A + b → ab. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. The general form of a synthesis reaction is: A combination reaction, also known as a synthesis. Synthesis Non Example Chemistry.
From royalsocietypublishing.org
Organic synthesis the art and science of replicating the molecules of Synthesis Non Example Chemistry The construction of complex and defined new molecules is a challenging and complicated. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. A combination reaction, also known as a synthesis reaction, is a reaction in which two or more substances combine to form a single new substance. The general form of a. Synthesis Non Example Chemistry.
From www.haikudeck.com
Synthesis Reaction by Natalie Gayed Synthesis Non Example Chemistry Chemists synthesize chemical compounds that occur in nature in order to gain a better understanding of their structures. The construction of complex and defined new molecules is a challenging and complicated. A + b → ab. General form of synthesis reactions. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple. Synthesis Non Example Chemistry.
From thechemistrynotes.com
Synthesis of Polyester Important Polymerization Reaction Synthesis Non Example Chemistry A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. Chemists synthesize chemical compounds that occur in nature in order to gain a better understanding of their structures. A synthesis reaction or direct combination reaction is one of the most common types of. Synthesis Non Example Chemistry.
From community.thechicagoschool.edu
Synthesis The Chicago School Community Synthesis Non Example Chemistry A + b → ab. Synthesis is the production of chemical compounds by reaction from simpler materials. A combination reaction, also known as a synthesis reaction, is a reaction in which two or more substances combine to form a single new substance. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. The. Synthesis Non Example Chemistry.
From chemistry.cas.lehigh.edu
Research chemistry Synthesis Non Example Chemistry Synthesis is the production of chemical compounds by reaction from simpler materials. A combination reaction, also known as a synthesis reaction, is a reaction in which two or more substances combine to form a single new substance. The construction of complex and defined new molecules is a challenging and complicated. General form of synthesis reactions. Chemists synthesize chemical compounds that. Synthesis Non Example Chemistry.
From thechemistrynotes.com
Organic Synthesis Strategies, Types Synthesis Non Example Chemistry Chemists synthesize chemical compounds that occur in nature in order to gain a better understanding of their structures. General form of synthesis reactions. The reactants may be elements or compounds, while the product is always a compound. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form. Synthesis Non Example Chemistry.
From www.zswu.dicp.ac.cn
Synthesis & Assembly Chemistry of 2D Materials二维材料化学与能源应用研究组 Synthesis Non Example Chemistry The reactants may be elements or compounds, while the product is always a compound. The general form of a synthesis reaction is: General form of synthesis reactions. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. Chemists synthesize chemical compounds that occur in nature in order to gain a better understanding of. Synthesis Non Example Chemistry.
From chemistry.stackexchange.com
organic chemistry Alternate pathway of Gabriel synthesis to prepare Synthesis Non Example Chemistry The general form of a synthesis reaction is: A + b → ab. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. Chemists synthesize chemical compounds that occur in nature in order to gain a better understanding of their structures. A combination reaction, also known as a synthesis reaction, is a reaction. Synthesis Non Example Chemistry.
From leah4sci.com
How to Tackle Organic Chemistry Synthesis Questions Organic Chemistry Synthesis Non Example Chemistry A + b → ab. The reactants may be elements or compounds, while the product is always a compound. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a. Synthesis Non Example Chemistry.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation ID6634764 Synthesis Non Example Chemistry Chemists synthesize chemical compounds that occur in nature in order to gain a better understanding of their structures. Synthesis is the production of chemical compounds by reaction from simpler materials. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. General form of synthesis reactions. A combination reaction, also known as a synthesis. Synthesis Non Example Chemistry.
From www.a-levelnotes.co.uk
Alevel Chemistry AQA Notes Organic Synthesis (ALevel) ALEVEL NOTES Synthesis Non Example Chemistry A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. A combination reaction, also known as a synthesis reaction, is a reaction in which two or more substances combine to form a single new substance. Synthesis is the production of chemical compounds by. Synthesis Non Example Chemistry.
From pdfprof.com
synthesis problems and answers Synthesis Non Example Chemistry Synthesis is the production of chemical compounds by reaction from simpler materials. Chemists synthesize chemical compounds that occur in nature in order to gain a better understanding of their structures. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. The construction of complex and defined new molecules is a challenging and complicated.. Synthesis Non Example Chemistry.
From collegeessay.org
12+ Synthesis Essay Examples to Inspire You Synthesis Non Example Chemistry A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. Chemists synthesize chemical compounds that occur in nature in order to gain a better understanding of. Synthesis Non Example Chemistry.
From www.reddit.com
Thought I'd share my organic synthesis cheat sheet I made during A Synthesis Non Example Chemistry A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. A combination reaction, also known as a synthesis reaction, is a reaction in which two or more substances combine to form a single new substance. Synthesis is the production of chemical compounds by. Synthesis Non Example Chemistry.
From www.thoughtco.com
Types of Chemical Reactions (With Examples) Synthesis Non Example Chemistry A combination reaction, also known as a synthesis reaction, is a reaction in which two or more substances combine to form a single new substance. Chemists synthesize chemical compounds that occur in nature in order to gain a better understanding of their structures. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or. Synthesis Non Example Chemistry.
From www.studocu.com
Synthesis CHEMICAL REACTION A synthesis reaction or direct Synthesis Non Example Chemistry A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. Synthesis is the production of chemical compounds by reaction from simpler materials. A + b → ab. A synthesis reaction or direct combination reaction is one of the most common types of chemical. Synthesis Non Example Chemistry.
From studylib.net
How to write a Synthesis Essay Synthesis Non Example Chemistry The construction of complex and defined new molecules is a challenging and complicated. The reactants may be elements or compounds, while the product is always a compound. Synthesis is the production of chemical compounds by reaction from simpler materials. A + b → ab. A synthesis reaction or direct combination reaction is one of the most common types of chemical. Synthesis Non Example Chemistry.
From custom-writing.org
How to Write a Synthesis Essay Examples, Topics, & Synthesis Essay Outline Synthesis Non Example Chemistry A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. Chemists synthesize chemical compounds that occur in nature in order to gain a better understanding of their structures. The reactants may be elements or compounds, while the product is always a compound. The. Synthesis Non Example Chemistry.
From www.conquerhsc.com
HSC Chemistry Module 7 Inquiry Question 5 Synthesis Non Example Chemistry Synthesis is the production of chemical compounds by reaction from simpler materials. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. A + b → ab. Chemists synthesize chemical compounds that occur in nature in order to gain a better understanding of their structures. The general form of a synthesis reaction is:. Synthesis Non Example Chemistry.
From www.chegg.com
Solved Organic Chemistry Synthesis practice Part 1 I Synthesis Non Example Chemistry The reactants may be elements or compounds, while the product is always a compound. A combination reaction, also known as a synthesis reaction, is a reaction in which two or more substances combine to form a single new substance. General form of synthesis reactions. The construction of complex and defined new molecules is a challenging and complicated. The general form. Synthesis Non Example Chemistry.
From www.pinterest.co.uk
Synthesis Chemistry Chemistry, Synthesis, Chemical structure Synthesis Non Example Chemistry A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. Synthesis is the production of chemical compounds by reaction from simpler materials. General form of synthesis reactions. A combination reaction, also known as a synthesis reaction, is a reaction in which two or. Synthesis Non Example Chemistry.
From webapi.bu.edu
A synthesis. Synthesis. 20221029 Synthesis Non Example Chemistry Synthesis is the production of chemical compounds by reaction from simpler materials. The construction of complex and defined new molecules is a challenging and complicated. Chemists synthesize chemical compounds that occur in nature in order to gain a better understanding of their structures. A + b → ab. General form of synthesis reactions. The reactants may be elements or compounds,. Synthesis Non Example Chemistry.
From www.pinterest.jp
[73] Gabriel Synthesis 1887 Organic chemistry books, Organic Synthesis Non Example Chemistry A + b → ab. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. The general form of a synthesis reaction is: A combination reaction, also known as a synthesis reaction, is a reaction in which two or more substances combine to form a single new substance. A synthesis reaction or direct. Synthesis Non Example Chemistry.
From www.mdpi.com
Applied Sciences Free FullText Aqueous MicrowaveAssisted Solid Synthesis Non Example Chemistry Synthesis is the production of chemical compounds by reaction from simpler materials. Chemists synthesize chemical compounds that occur in nature in order to gain a better understanding of their structures. The general form of a synthesis reaction is: A combination reaction, also known as a synthesis reaction, is a reaction in which two or more substances combine to form a. Synthesis Non Example Chemistry.
From www.pinterest.com
What Is a Synthesis Reaction? Definition and Examples in 2021 Synthesis Non Example Chemistry The construction of complex and defined new molecules is a challenging and complicated. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. Synthesis is the production of chemical compounds by reaction from simpler materials. General form of synthesis reactions. The reactants may. Synthesis Non Example Chemistry.
From www.pdfprof.com
synthesis problems and answers Synthesis Non Example Chemistry The reactants may be elements or compounds, while the product is always a compound. Synthesis is the production of chemical compounds by reaction from simpler materials. Chemists synthesize chemical compounds that occur in nature in order to gain a better understanding of their structures. A + b → ab. A synthesis reaction or direct combination reaction is a type of. Synthesis Non Example Chemistry.
From www.examples.com
Synthesis Essay 20+ Examples, Structure, How to Write, Tips, Pdf Synthesis Non Example Chemistry The construction of complex and defined new molecules is a challenging and complicated. A + b → ab. General form of synthesis reactions. Chemists synthesize chemical compounds that occur in nature in order to gain a better understanding of their structures. The reactants may be elements or compounds, while the product is always a compound. The general form of a. Synthesis Non Example Chemistry.
From gamesmartz.com
Synthesis Definition & Image GameSmartz Synthesis Non Example Chemistry A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. A + b → ab. The reactants may be elements or compounds, while the product is always a compound. The construction of complex and defined new molecules is a challenging and complicated. Synthesis. Synthesis Non Example Chemistry.