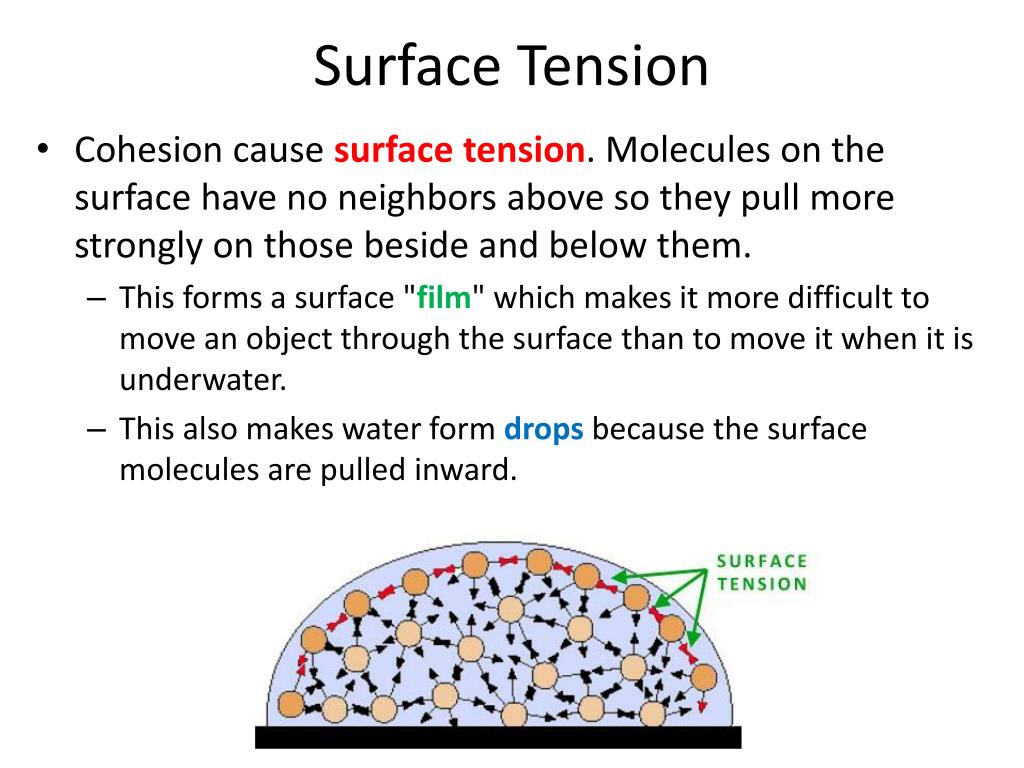
What Does Surface Tension Affect . “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Why do bubbles form in a glass of water? Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. This effect results from the difference between the potential energy. Since these intermolecular forces vary depending on the nature of the. Surface tension may be expressed, therefore, in units of energy per unit area (square metres).
from www.slideserve.com
Why do bubbles form in a glass of water? “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. Since these intermolecular forces vary depending on the nature of the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). This effect results from the difference between the potential energy. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one.
PPT Unit 4 The Hydrosphere PowerPoint Presentation, free download
What Does Surface Tension Affect “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. This effect results from the difference between the potential energy. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Why do bubbles form in a glass of water? “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Since these intermolecular forces vary depending on the nature of the.
From www.bigstockphoto.com
Surface Tension Image & Photo (Free Trial) Bigstock What Does Surface Tension Affect Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends. What Does Surface Tension Affect.
From ar.inspiredpencil.com
Surface Tension Of Water Diagram What Does Surface Tension Affect Since these intermolecular forces vary depending on the nature of the. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. “surface tension is the tension of a liquid’s surface film caused by the bulk. What Does Surface Tension Affect.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 What Does Surface Tension Affect Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Surface tension is the energy, or work, required to increase the. What Does Surface Tension Affect.
From scienceinfo.com
Surface Tension Definition, Formula, Unit, Causes, Examples, Consequences What Does Surface Tension Affect “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. This effect results from the difference between the potential energy. Since these intermolecular forces vary depending on the nature of the. Water has a surface tension of 0.07275 joule. What Does Surface Tension Affect.
From za.pinterest.com
How does Surface Tension work? Surface tension, Tension physics What Does Surface Tension Affect Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Why do bubbles form in a glass of water? This effect results from the difference between the potential energy. “surface tension is the tension of a liquid’s. What Does Surface Tension Affect.
From www.worksheetsplanet.com
What is Surface Tension? What Does Surface Tension Affect Why do bubbles form in a glass of water? Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Surface tension. What Does Surface Tension Affect.
From readchemistry.com
Determination of Surface Tension Read Chemistry What Does Surface Tension Affect Since these intermolecular forces vary depending on the nature of the. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. “surface tension is the tension of a liquid’s surface film caused. What Does Surface Tension Affect.
From www.healthbenefitstimes.com
Surface tension Definition of Surface tension What Does Surface Tension Affect Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. Since these intermolecular forces vary depending on the nature of the. Why do bubbles form in a glass of water? Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one. This effect results from the difference between. What Does Surface Tension Affect.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL What Does Surface Tension Affect “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Why do bubbles form in a glass of water? This effect results from the difference between the potential energy. Simply put, surface tension is the tendency of molecules of. What Does Surface Tension Affect.
From www.biolinscientific.com
Surface tension of water Why is it so high? What Does Surface Tension Affect Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one. Since these intermolecular forces vary depending on the nature of the. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Surface tension is the energy, or work, required to increase the surface area of a liquid. What Does Surface Tension Affect.
From littletogreatscientists.com
What is Surface Tension? Little to Great Scientists What Does Surface Tension Affect Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Since these intermolecular forces vary depending on the nature. What Does Surface Tension Affect.
From byjus.com
Explain the surface tension phenomenon with examples. What Does Surface Tension Affect Since these intermolecular forces vary depending on the nature of the. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Simply put, surface. What Does Surface Tension Affect.
From www.moleaer.com
How nanobubbles improve water infiltration by reducing surface tension What Does Surface Tension Affect Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Why do bubbles form in a glass of water? Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one. This effect results from the difference between the potential energy. Surface tension is the energy, or work, required. What Does Surface Tension Affect.
From www.linkedin.com
What is Surface Tension? What Does Surface Tension Affect Since these intermolecular forces vary depending on the nature of the. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Why do bubbles form in a glass of water? Simply put, surface tension is the tendency of molecules of. What Does Surface Tension Affect.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector What Does Surface Tension Affect Why do bubbles form in a glass of water? Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the. Water has. What Does Surface Tension Affect.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy What Does Surface Tension Affect Since these intermolecular forces vary depending on the nature of the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. This effect results from the difference between the potential energy. “surface tension is the. What Does Surface Tension Affect.
From worksheethaunches.z14.web.core.windows.net
Surface Tension Explained For Kids What Does Surface Tension Affect Why do bubbles form in a glass of water? “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. Since these intermolecular forces vary. What Does Surface Tension Affect.
From civilquery.com
What is Surface Tension Definition, Examples and Tests Civil Query What Does Surface Tension Affect Since these intermolecular forces vary depending on the nature of the. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one. This effect results from the difference between the potential energy. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. “surface. What Does Surface Tension Affect.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments What Does Surface Tension Affect Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. This effect results from the difference between the potential energy. Since these intermolecular forces vary depending on the nature of the. Why do bubbles form in a glass of water? Surface tension is the energy, or work, required to increase the surface area of a. What Does Surface Tension Affect.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical What Does Surface Tension Affect Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Since these intermolecular forces vary depending on the nature of the. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Water has a. What Does Surface Tension Affect.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance What Does Surface Tension Affect Why do bubbles form in a glass of water? “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. Surface tension may be expressed,. What Does Surface Tension Affect.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog What Does Surface Tension Affect Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. Since these intermolecular forces vary depending on the nature of the. This effect results from the difference between the potential energy. Why do bubbles form. What Does Surface Tension Affect.
From www.youtube.com
Surface Tension on the basis of Molecular theory YouTube What Does Surface Tension Affect Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. This effect results from the difference between the potential energy. Why do bubbles form. What Does Surface Tension Affect.
From cesioues.blob.core.windows.net
High Surface Tension Facts at Margaret Halpern blog What Does Surface Tension Affect Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. This effect results from the difference between the potential energy. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the. What Does Surface Tension Affect.
From study.com
Surface Tension Definition, Calculation & Examples Video & Lesson What Does Surface Tension Affect Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Why do bubbles form in a glass of water? This. What Does Surface Tension Affect.
From www.slideserve.com
PPT Unit 4 The Hydrosphere PowerPoint Presentation, free download What Does Surface Tension Affect This effect results from the difference between the potential energy. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Why do bubbles form in a glass of water? Since these intermolecular. What Does Surface Tension Affect.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and What Does Surface Tension Affect Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). This effect results from the difference between the potential energy. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s. What Does Surface Tension Affect.
From chem.libretexts.org
11.4 Intermolecular Forces in Action Surface Tension, Viscosity, and What Does Surface Tension Affect Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Why do bubbles form in a glass of water? “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Since these intermolecular forces vary. What Does Surface Tension Affect.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it What Does Surface Tension Affect “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Since these intermolecular forces vary depending on the nature of the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to. What Does Surface Tension Affect.
From www.studypool.com
SOLUTION Factors affecting surface tension Studypool What Does Surface Tension Affect Why do bubbles form in a glass of water? “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Since these intermolecular forces vary. What Does Surface Tension Affect.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance What Does Surface Tension Affect Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. Why do bubbles form in a glass of water? Surface tension may be expressed, therefore, in units of energy per unit area (square metres). “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles. What Does Surface Tension Affect.
From www.merchantnavydecoded.com
What is Surface tension? Why does surface tension occur? Merchant What Does Surface Tension Affect Surface tension may be expressed, therefore, in units of energy per unit area (square metres). “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Since these intermolecular forces vary depending on the nature of the. Why do bubbles. What Does Surface Tension Affect.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids What Does Surface Tension Affect Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Why do bubbles form in a glass of water? “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”.. What Does Surface Tension Affect.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint What Does Surface Tension Affect “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. This effect results from the difference between the potential energy. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. Surface tension is the. What Does Surface Tension Affect.
From kidsactivitiesblog.com
What is Surface Tension? Simple Science Activity for Kids Kids What Does Surface Tension Affect Since these intermolecular forces vary depending on the nature of the. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. Why do bubbles form in a glass of water? Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one. Surface tension may be expressed, therefore, in. What Does Surface Tension Affect.