Bromine Water Mechanism . When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. If you have a gaseous alkene like ethene, you can bubble it through either pure. During the first step of the mechanism, the 2 pi electrons from the double bond attack the h in the hbr. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The generalised mechanism by a halogenated alcohol is formed when an alkene reacts with bromine water. The reason form the formation of the halogenated alcohol is explained via the. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction with bromine happens at room temperature. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless.
from www.masterorganicchemistry.com
During the first step of the mechanism, the 2 pi electrons from the double bond attack the h in the hbr. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. The generalised mechanism by a halogenated alcohol is formed when an alkene reacts with bromine water. The reaction with bromine happens at room temperature. The reason form the formation of the halogenated alcohol is explained via the. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. If you have a gaseous alkene like ethene, you can bubble it through either pure.
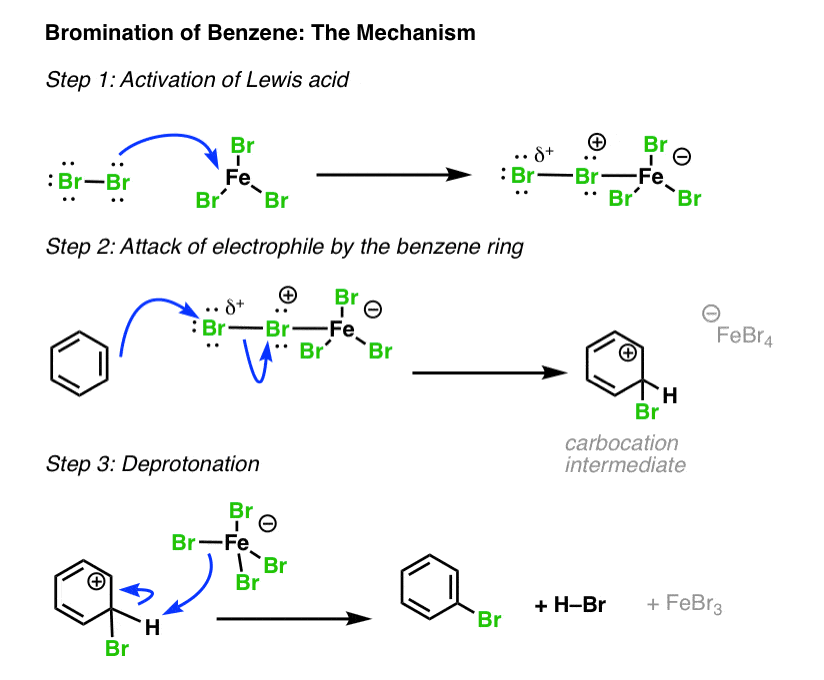
Electrophilic Aromatic Substitutions Chlorination and Bromination
Bromine Water Mechanism The reaction with bromine happens at room temperature. The reason form the formation of the halogenated alcohol is explained via the. The generalised mechanism by a halogenated alcohol is formed when an alkene reacts with bromine water. The reaction with bromine happens at room temperature. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. During the first step of the mechanism, the 2 pi electrons from the double bond attack the h in the hbr. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. If you have a gaseous alkene like ethene, you can bubble it through either pure. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample.
From www.teachoo.com
Unsaturated hydrocarbons contain multiple bonds between 2 carbon atoms Bromine Water Mechanism Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. During the first step of the mechanism, the 2 pi electrons from the double bond attack the h in the hbr. The reason form the formation of the halogenated alcohol is explained via the. Alkenes react in the cold. Bromine Water Mechanism.
From gradegorilla.com
GCSE Chemistry Organic Grade Gorilla Bromine Water Mechanism If you have a gaseous alkene like ethene, you can bubble it through either pure. The reaction with bromine happens at room temperature. During the first step of the mechanism, the 2 pi electrons from the double bond attack the h in the hbr. When bromine is added to the sample, if the reddish color disappear, that means the sample. Bromine Water Mechanism.
From www.masterorganicchemistry.com
Bromination of alkenes with Br2 to give dibromides Master Organic Bromine Water Mechanism The reason form the formation of the halogenated alcohol is explained via the. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. During the first step of the mechanism, the 2 pi electrons from the double bond attack the h in the hbr. The generalised mechanism by a halogenated. Bromine Water Mechanism.
From www.youtube.com
Bromine Water Experiment YouTube Bromine Water Mechanism The generalised mechanism by a halogenated alcohol is formed when an alkene reacts with bromine water. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an. Bromine Water Mechanism.
From mungfali.com
Ethene And Water Reaction Bromine Water Mechanism If you have a gaseous alkene like ethene, you can bubble it through either pure. The reason form the formation of the halogenated alcohol is explained via the. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Alkenes react in the cold with pure liquid bromine, or with a. Bromine Water Mechanism.
From www.youtube.com
4. Bromine water experiment (HSC chemistry) YouTube Bromine Water Mechanism During the first step of the mechanism, the 2 pi electrons from the double bond attack the h in the hbr. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain. Bromine Water Mechanism.
From chem.libretexts.org
10.4 Reactions of Alkenes Addition of Bromine and Chlorine to Alkenes Bromine Water Mechanism If you have a gaseous alkene like ethene, you can bubble it through either pure. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction between. Bromine Water Mechanism.
From franklychemistry.co.uk
Ethene with Bromine Bromine Water Mechanism During the first step of the mechanism, the 2 pi electrons from the double bond attack the h in the hbr. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The reason form the formation of the halogenated alcohol is explained via the. When. Bromine Water Mechanism.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation Bromine Water Mechanism Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction with bromine happens at room temperature. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction between c=c double bond and bromine (br 2). Bromine Water Mechanism.
From www.researchgate.net
Optimization of bromination. 1 Download Scientific Diagram Bromine Water Mechanism The generalised mechanism by a halogenated alcohol is formed when an alkene reacts with bromine water. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. During the first step of the mechanism, the 2 pi. Bromine Water Mechanism.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation Bromine Water Mechanism Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction with bromine happens at room temperature. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. If you have a gaseous alkene. Bromine Water Mechanism.
From www.dev.odinity.com
Stereochemistry of Bromine Addition to an Alkene Bromine Water Mechanism Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. During the first step of the mechanism, the 2 pi electrons from the double bond attack the h in the hbr. The reaction with bromine happens at room temperature. The generalised mechanism by a halogenated alcohol is formed when. Bromine Water Mechanism.
From www.chemtube3d.com
BaseCatalysed Bromination of Ketones Summary Bromine Water Mechanism The reason form the formation of the halogenated alcohol is explained via the. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. During the first step of the. Bromine Water Mechanism.
From www.youtube.com
Bromine Addition Mechanism to Fumaric Acid Formation of meso bromine Bromine Water Mechanism If you have a gaseous alkene like ethene, you can bubble it through either pure. The generalised mechanism by a halogenated alcohol is formed when an alkene reacts with bromine water. The reason form the formation of the halogenated alcohol is explained via the. When bromine is added to the sample, if the reddish color disappear, that means the sample. Bromine Water Mechanism.
From dawson-bogspotsheppard.blogspot.com
Bromine Water Test Equation Bromine Water Mechanism When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. The. Bromine Water Mechanism.
From www.slideserve.com
PPT OCR organic reaction mechanisms PowerPoint Presentation, free Bromine Water Mechanism If you have a gaseous alkene like ethene, you can bubble it through either pure. The generalised mechanism by a halogenated alcohol is formed when an alkene reacts with bromine water. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction between c=c double bond and bromine. Bromine Water Mechanism.
From www.youtube.com
Reactions with Bromine Water YouTube Bromine Water Mechanism The generalised mechanism by a halogenated alcohol is formed when an alkene reacts with bromine water. The reaction with bromine happens at room temperature. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine. Bromine Water Mechanism.
From www.masterorganicchemistry.com
Electrophilic Aromatic Substitutions Chlorination and Bromination Bromine Water Mechanism Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. The reason form the. Bromine Water Mechanism.
From chem.libretexts.org
10.4 Reactions of Alkenes Addition of Bromine and Chlorine to Alkenes Bromine Water Mechanism The reason form the formation of the halogenated alcohol is explained via the. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. During the first step of the mechanism, the. Bromine Water Mechanism.
From www.coursehero.com
[Solved] The mechanism shows how a solution of bromine in water reacts Bromine Water Mechanism The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine. Bromine Water Mechanism.
From www.masterorganicchemistry.com
Bromination of Alkenes Master Organic Chemistry Bromine Water Mechanism If you have a gaseous alkene like ethene, you can bubble it through either pure. The reaction with bromine happens at room temperature. During the first step of the mechanism, the 2 pi electrons from the double bond attack the h in the hbr. The reason form the formation of the halogenated alcohol is explained via the. Alkenes react in. Bromine Water Mechanism.
From www.youtube.com
Electrophilic Addition of Bromine to Ethene YouTube Bromine Water Mechanism If you have a gaseous alkene like ethene, you can bubble it through either pure. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. The reaction with bromine happens at room temperature. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The generalised. Bromine Water Mechanism.
From www.vedantu.com
Reaction of bromine water phenol gives white ppt ofA. o bromophenolB Bromine Water Mechanism Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction with bromine happens at room temperature. If you have a gaseous alkene like ethene, you can bubble it through either pure. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. The reason. Bromine Water Mechanism.
From www.doubtnut.com
Explain the action of bromine water on phenol (carbolic acid). Bromine Water Mechanism The reaction with bromine happens at room temperature. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. If you have a gaseous alkene like ethene, you can bubble it through either pure. The reaction between c=c double bond and bromine (br 2) can be used as a test. Bromine Water Mechanism.
From byjus.com
What happens when ethane reacts with bromine water? Bromine Water Mechanism Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction with bromine happens at room temperature. The reason form the formation of the halogenated alcohol is explained via the. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain. Bromine Water Mechanism.
From www.youtube.com
Electrophilic Addition Mechanism with Bromine YouTube Bromine Water Mechanism If you have a gaseous alkene like ethene, you can bubble it through either pure. The generalised mechanism by a halogenated alcohol is formed when an alkene reacts with bromine water. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. The reason form the formation of the halogenated alcohol. Bromine Water Mechanism.
From www.youtube.com
Bromination of cyclohexene YouTube Bromine Water Mechanism During the first step of the mechanism, the 2 pi electrons from the double bond attack the h in the hbr. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent. Bromine Water Mechanism.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 4.4.2 Bromine & Alkenes翰林国际教育 Bromine Water Mechanism Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reason form the formation of the halogenated alcohol is explained via the. During the first step of the mechanism, the 2 pi electrons from the double bond attack the h in the hbr. If you have a gaseous. Bromine Water Mechanism.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation Bromine Water Mechanism During the first step of the mechanism, the 2 pi electrons from the double bond attack the h in the hbr. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain. Bromine Water Mechanism.
From chemistryguru.com.sg
Electrophilic Addition with Aqueous Bromine Bromine Water Mechanism The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. If you have a gaseous alkene like ethene, you can bubble it through either pure. The generalised mechanism by a halogenated alcohol is formed when. Bromine Water Mechanism.
From www.masterorganicchemistry.com
Bromination of Alkenes The Mechanism Master Organic Chemistry Bromine Water Mechanism Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. If you have a gaseous alkene like ethene, you can bubble it through either pure. The reason form the formation of the halogenated alcohol is explained via the. When bromine is added to the sample, if the reddish color. Bromine Water Mechanism.
From www.youtube.com
Bromine Water Test Explained// HSC Chemistry YouTube Bromine Water Mechanism The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. If you have a gaseous alkene like ethene, you can bubble it through either pure. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene.. Bromine Water Mechanism.
From pdfprof.com
carboxylic acid reaction with bromine water Bromine Water Mechanism Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. When bromine is added to the sample, if the reddish color disappear, that means. Bromine Water Mechanism.
From crunchchemistry.co.uk
Electrophilic addition in alkenes (2) the bromine water test Crunch Bromine Water Mechanism The reason form the formation of the halogenated alcohol is explained via the. The generalised mechanism by a halogenated alcohol is formed when an alkene reacts with bromine water. The reaction with bromine happens at room temperature. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction. Bromine Water Mechanism.
From brainly.in
phenol gives 2,4,6tribromophenol oN bromination with bromine water Bromine Water Mechanism During the first step of the mechanism, the 2 pi electrons from the double bond attack the h in the hbr. The reason form the formation of the halogenated alcohol is explained via the. The reaction with bromine happens at room temperature. The reaction between c=c double bond and bromine (br 2) can be used as a test for the. Bromine Water Mechanism.