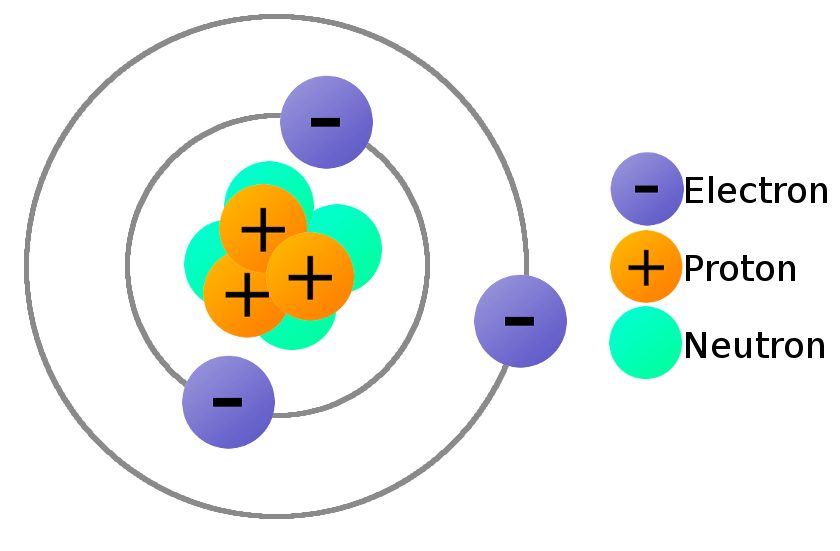
Do Atoms Have No Charge . But sometimes an atom can gain or lose an electron to become what's called an ion. Protons have a +1 charge, and. Atoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons. An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons. Since an atom contains equal number of protons and electrons, it has no overall charge: Relative charges of −1 and +1 are assigned to the electron and proton, respectively. Neutrons have approximately the same mass as protons but no charge. Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. An atom has no overall charge because each element has the same number of protons and electrons. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons.
from fablabbcn-projects.gitlab.io
Relative charges of −1 and +1 are assigned to the electron and proton, respectively. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. Protons have a +1 charge, and. Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons. But sometimes an atom can gain or lose an electron to become what's called an ion. Neutrons have approximately the same mass as protons but no charge. Atoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons. An atom has no overall charge because each element has the same number of protons and electrons. Since an atom contains equal number of protons and electrons, it has no overall charge:
Day 1 Fablab BCN Local Documentation
Do Atoms Have No Charge Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. But sometimes an atom can gain or lose an electron to become what's called an ion. Protons have a +1 charge, and. Neutrons have approximately the same mass as protons but no charge. An atom has no overall charge because each element has the same number of protons and electrons. Relative charges of −1 and +1 are assigned to the electron and proton, respectively. An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons. Since an atom contains equal number of protons and electrons, it has no overall charge: Atoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons.
From classfullplymouth.z21.web.core.windows.net
Parts Of The Atom Do Atoms Have No Charge Relative charges of −1 and +1 are assigned to the electron and proton, respectively. An atom has no overall charge because each element has the same number of protons and electrons. Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. The positive charges on all the protons are exactly balanced by the negative charges. Do Atoms Have No Charge.
From slideplayer.com
Atoms Vocab. ppt download Do Atoms Have No Charge Protons have a +1 charge, and. Atoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons. An atom has no overall charge because each element has the same number of protons and electrons. Neutrons have approximately the same mass as protons but no charge. Electrons and protons have electrical charges that are. Do Atoms Have No Charge.
From www.slideserve.com
PPT What is Matter? PowerPoint Presentation, free download ID5074624 Do Atoms Have No Charge Atoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. An atom has no overall charge because each element has the same number of protons and electrons. Neutrons have approximately the same mass as. Do Atoms Have No Charge.
From www.slideserve.com
PPT Atomic Theorists PowerPoint Presentation, free download ID1255014 Do Atoms Have No Charge Relative charges of −1 and +1 are assigned to the electron and proton, respectively. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. But sometimes an atom can gain or lose an electron to become what's called an ion. An atom consists of a positively charged nucleus, surrounded by one or. Do Atoms Have No Charge.
From slideplayer.com
Atoms and the Periodic Table. ppt download Do Atoms Have No Charge Atoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons. Neutrons have approximately the same mass as protons but no charge. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. Since an atom contains equal number of protons and electrons, it has no. Do Atoms Have No Charge.
From slideplayer.com
Bell Work Review your notes on the atomic theory. ppt download Do Atoms Have No Charge The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Atoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons. An atom consists of a positively charged nucleus, surrounded. Do Atoms Have No Charge.
From fablabbcn-projects.gitlab.io
Day 1 Fablab BCN Local Documentation Do Atoms Have No Charge The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. Relative charges of −1 and +1 are assigned to the electron and proton, respectively. Since an atom contains equal number of protons and electrons, it has no overall charge: But sometimes an atom can gain or lose an electron to become what's. Do Atoms Have No Charge.
From www.slideserve.com
PPT Atoms and the Periodic Table PowerPoint Presentation, free Do Atoms Have No Charge But sometimes an atom can gain or lose an electron to become what's called an ion. Neutrons have approximately the same mass as protons but no charge. Relative charges of −1 and +1 are assigned to the electron and proton, respectively. Atoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons. An. Do Atoms Have No Charge.
From slideplayer.com
What’s an XRay Ionization. ppt download Do Atoms Have No Charge Protons have a +1 charge, and. Atoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons. An atom has no overall charge because each element has the same number of protons and electrons. But sometimes an atom can gain or lose an electron to become what's called an ion. Relative charges of. Do Atoms Have No Charge.
From www.youtube.com
1.4 Explaining why atoms have no overall charge. YouTube Do Atoms Have No Charge Neutrons have approximately the same mass as protons but no charge. Protons have a +1 charge, and. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons. An atom has no overall charge because. Do Atoms Have No Charge.
From www.slideserve.com
PPT Chapter 3 Atoms and the Periodic Table PowerPoint Presentation Do Atoms Have No Charge Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Atoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons. Since an atom contains equal number of protons and electrons, it has no overall charge: The positive charges on all the protons are exactly balanced by the. Do Atoms Have No Charge.
From slideplayer.com
Atoms Vocab. ppt download Do Atoms Have No Charge Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Since an atom contains equal number of protons and electrons, it has no overall charge: Relative charges of −1 and +1 are assigned to the electron and proton, respectively. But sometimes an atom can gain or lose an electron to become what's called an ion.. Do Atoms Have No Charge.
From slideplayer.com
What are Atoms? Atoms are tiny subunits that determine the properties Do Atoms Have No Charge Protons have a +1 charge, and. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Neutrons have approximately the same mass as protons but no charge. Relative charges of −1 and +1 are assigned to the. Do Atoms Have No Charge.
From diagrampartnumerosity.z21.web.core.windows.net
Diagram Of The Atom Do Atoms Have No Charge Relative charges of −1 and +1 are assigned to the electron and proton, respectively. But sometimes an atom can gain or lose an electron to become what's called an ion. Neutrons have approximately the same mass as protons but no charge. Since an atom contains equal number of protons and electrons, it has no overall charge: Protons have a +1. Do Atoms Have No Charge.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts Do Atoms Have No Charge An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons. Atoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons. Protons have a +1 charge, and. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons.. Do Atoms Have No Charge.
From www.slideserve.com
PPT Atoms have NO overall charge PowerPoint Presentation, free Do Atoms Have No Charge An atom has no overall charge because each element has the same number of protons and electrons. Neutrons have approximately the same mass as protons but no charge. Protons have a +1 charge, and. An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons. Relative charges of −1 and +1 are assigned. Do Atoms Have No Charge.
From leverageedu.com
Structure Of An Atom Class 9 Science Notes Leverage Edu Do Atoms Have No Charge Neutrons have approximately the same mass as protons but no charge. An atom has no overall charge because each element has the same number of protons and electrons. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. Relative charges of −1 and +1 are assigned to the electron and proton, respectively.. Do Atoms Have No Charge.
From www.slideserve.com
PPT 11 th grade Physical Science Chapter 4 Notes PowerPoint Do Atoms Have No Charge Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Since an atom contains equal number of protons and electrons, it has no overall charge: Protons have a +1 charge, and. But sometimes an atom can gain or lose an electron to become what's called an ion. Neutrons have approximately the same mass as protons. Do Atoms Have No Charge.
From www.slidemake.com
Structure Of Atom Presentation Do Atoms Have No Charge But sometimes an atom can gain or lose an electron to become what's called an ion. Relative charges of −1 and +1 are assigned to the electron and proton, respectively. An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons. Neutrons have approximately the same mass as protons but no charge. An. Do Atoms Have No Charge.
From www.thesciencehive.co.uk
Atomic Structure* — the science sauce Do Atoms Have No Charge An atom has no overall charge because each element has the same number of protons and electrons. Protons have a +1 charge, and. Neutrons have approximately the same mass as protons but no charge. Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. The positive charges on all the protons are exactly balanced by. Do Atoms Have No Charge.
From www.nagwa.com
Question Video Explaining Why Atoms Do Not Have a Charge Nagwa Do Atoms Have No Charge An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons. Atoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons. But sometimes an atom can gain or lose an electron to become what's called an ion. Since an atom contains equal number of protons. Do Atoms Have No Charge.
From www.slideserve.com
PPT Atomic Theorists PowerPoint Presentation, free download ID1255014 Do Atoms Have No Charge Protons have a +1 charge, and. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons. Since an atom contains equal number of protons and electrons, it has no overall charge: But sometimes an. Do Atoms Have No Charge.
From www.broadlearnings.com
Atomic Structure Broad Learnings Do Atoms Have No Charge But sometimes an atom can gain or lose an electron to become what's called an ion. Protons have a +1 charge, and. An atom has no overall charge because each element has the same number of protons and electrons. Atoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons. Electrons and protons. Do Atoms Have No Charge.
From garagerepairalbacore.z14.web.core.windows.net
Label The Parts Of An Atom On The Diagram Do Atoms Have No Charge Relative charges of −1 and +1 are assigned to the electron and proton, respectively. Neutrons have approximately the same mass as protons but no charge. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. An atom has no overall charge because each element has the same number of protons and electrons.. Do Atoms Have No Charge.
From www.science-revision.co.uk
Atoms to ions Do Atoms Have No Charge Since an atom contains equal number of protons and electrons, it has no overall charge: The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. Relative charges of −1 and +1 are assigned to the electron and proton, respectively. Protons have a +1 charge, and. Atoms are electrically neutral if they contain. Do Atoms Have No Charge.
From www.youtube.com
Why atoms have no overall charge and ions do have a charge? YouTube Do Atoms Have No Charge An atom has no overall charge because each element has the same number of protons and electrons. But sometimes an atom can gain or lose an electron to become what's called an ion. Atoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons. Since an atom contains equal number of protons and. Do Atoms Have No Charge.
From electricallive.com
Atomic Structure And Electric Charge Electrical engineering interview Do Atoms Have No Charge Protons have a +1 charge, and. An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons. Neutrons have approximately the same mass as protons but no charge. But sometimes an atom can gain or lose an electron to become what's called an ion. Electrons and protons have electrical charges that are identical. Do Atoms Have No Charge.
From slideplayer.com
Atomic Number, Mass Number, Atomic Mass and Isotopes ppt download Do Atoms Have No Charge Atoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons. Since an atom contains equal number of protons and electrons, it has no overall charge: But sometimes an atom can gain or lose an electron to become what's called an ion. Protons have a +1 charge, and. The positive charges on all. Do Atoms Have No Charge.
From mungfali.com
Label The Parts Of An Atom Do Atoms Have No Charge Neutrons have approximately the same mass as protons but no charge. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons. But sometimes an atom can gain or lose an electron to become what's. Do Atoms Have No Charge.
From www.slideserve.com
PPT Atoms have NO overall charge PowerPoint Presentation, free Do Atoms Have No Charge Since an atom contains equal number of protons and electrons, it has no overall charge: The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. Atoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons. Electrons and protons have electrical charges that are identical. Do Atoms Have No Charge.
From www.pinterest.com
Spectroscopy Electron configuration, Chemistry education, Protons Do Atoms Have No Charge Protons have a +1 charge, and. But sometimes an atom can gain or lose an electron to become what's called an ion. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. Relative charges of −1 and +1 are assigned to the electron and proton, respectively. Neutrons have approximately the same mass. Do Atoms Have No Charge.
From www.slideserve.com
PPT Atoms have NO overall charge PowerPoint Presentation, free Do Atoms Have No Charge Neutrons have approximately the same mass as protons but no charge. Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Relative charges of −1 and +1 are assigned to the electron and proton, respectively. An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons. The positive. Do Atoms Have No Charge.
From slidetodoc.com
Atoms and the Periodic Table Chapter 4 Study Do Atoms Have No Charge But sometimes an atom can gain or lose an electron to become what's called an ion. Atoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons. Protons have a +1 charge, and. Relative charges of −1 and +1 are assigned to the electron and proton, respectively. The positive charges on all the. Do Atoms Have No Charge.
From slideplayer.com
Physical Science SPEEDY Midterm Review ppt download Do Atoms Have No Charge Since an atom contains equal number of protons and electrons, it has no overall charge: An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons. But sometimes an atom can gain or lose an electron to become what's called an ion. Protons have a +1 charge, and. Neutrons have approximately the same. Do Atoms Have No Charge.
From www.sliderbase.com
Atoms and the Periodic table Presentation Chemistry Do Atoms Have No Charge Neutrons have approximately the same mass as protons but no charge. An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons. Protons have a +1 charge, and. Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Relative charges of −1 and +1 are assigned to the. Do Atoms Have No Charge.